To Break or to Brake Neuronal Network Accelerated by Ammonium Ions?
- PMID: 26217943
- PMCID: PMC4517767
- DOI: 10.1371/journal.pone.0134145
To Break or to Brake Neuronal Network Accelerated by Ammonium Ions?
Abstract
Purpose: The aim of present study was to investigate the effects of ammonium ions on in vitro neuronal network activity and to search alternative methods of acute ammonia neurotoxicity prevention.
Methods: Rat hippocampal neuronal and astrocytes co-cultures in vitro, fluorescent microscopy and perforated patch clamp were used to monitor the changes in intracellular Ca2+- and membrane potential produced by ammonium ions and various modulators in the cells implicated in neural networks.
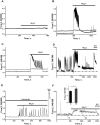
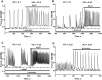
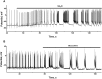
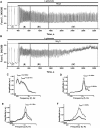
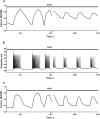
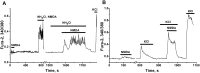
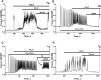
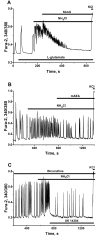
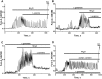
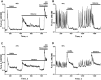
Results: Low concentrations of NH4Cl (0.1-4 mM) produce short temporal effects on network activity. Application of 5-8 mM NH4Cl: invariably transforms diverse network firing regimen to identical burst patterns, characterized by substantial neuronal membrane depolarization at plateau phase of potential and high-amplitude Ca2+-oscillations; raises frequency and average for period of oscillations Ca2+-level in all cells implicated in network; results in the appearance of group of «run out» cells with high intracellular Ca2+ and steadily diminished amplitudes of oscillations; increases astrocyte Ca2+-signalling, characterized by the appearance of groups of cells with increased intracellular Ca2+-level and/or chaotic Ca2+-oscillations. Accelerated network activity may be suppressed by the blockade of NMDA or AMPA/kainate-receptors or by overactivation of AMPA/kainite-receptors. Ammonia still activate neuronal firing in the presence of GABA(A) receptors antagonist bicuculline, indicating that «disinhibition phenomenon» is not implicated in the mechanisms of networks acceleration. Network activity may also be slowed down by glycine, agonists of metabotropic inhibitory receptors, betaine, L-carnitine, L-arginine, etc.
Conclusions: Obtained results demonstrate that ammonium ions accelerate neuronal networks firing, implicating ionotropic glutamate receptors, having preserved the activities of group of inhibitory ionotropic and metabotropic receptors. This may mean, that ammonia neurotoxicity might be prevented by the activation of various inhibitory receptors (i.e. by the reinforcement of negative feedback control), instead of application of various enzyme inhibitors and receptor antagonists (breaking of neural, metabolic and signaling systems).
Conflict of interest statement
Figures


References
-
- Sherlock S. Hepatic coma. Gastroenterology. Official publication of the American Gastroenterological Association. 1961;07: 1–8.
-
- Colombo JP. Congenital disorders of the urea cycle and ammonia detoxication. [Review]. Monogr Paediatr. 1971;1: 1–150. - PubMed
-
- Hindfelt B, Siesjo BK. Cerebral effects of acute ammonia intoxication. II. The effect upon energy metabolism. Scand J Clin Lab Invest. 1971;28: 365–74. - PubMed
-
- O'Connor JE, Costell M, Grisolia S. Protective effect of L-carnitine on hyperammonemia. FEBS Lett. 1984;166: 331–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

