Orally Administered Enoxaparin Ameliorates Acute Colitis by Reducing Macrophage-Associated Inflammatory Responses
- PMID: 26218284
- PMCID: PMC4517792
- DOI: 10.1371/journal.pone.0134259
Orally Administered Enoxaparin Ameliorates Acute Colitis by Reducing Macrophage-Associated Inflammatory Responses
Abstract
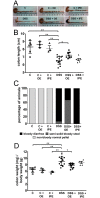
Inflammatory bowel diseases, such as ulcerative colitis, cause significant morbidity and decreased quality of life. The currently available treatments are not effective in all patients, can be expensive and have potential to cause severe side effects. This prompts the need for new treatment modalities. Enoxaparin, a widely used antithrombotic agent, is reported to possess anti-inflammatory properties and therefore we evaluated its therapeutic potential in a mouse model of colitis. Acute colitis was induced in male C57BL/6 mice by administration of dextran sulfate sodium (DSS). Mice were treated once daily with enoxaparin via oral or intraperitoneal administration and monitored for colitis activities. On termination (day 8), colons were collected for macroscopic evaluation and cytokine measurement, and processed for histology and immunohistochemistry. Oral but not intraperitoneal administration of enoxaparin significantly ameliorated DSS-induced colitis. Oral enoxaparin-treated mice retained their body weight and displayed less diarrhea and fecal blood loss compared to the untreated colitis group. Colon weight in enoxaparin-treated mice was significantly lower, indicating reduced inflammation and edema. Histological examination of untreated colitis mice showed a massive loss of crypt architecture and goblet cells, infiltration of immune cells and the presence of edema, while all aspects of this pathology were alleviated by oral enoxaparin. Reduced number of macrophages in the colon of oral enoxaparin-treated mice was accompanied by decreased levels of pro-inflammatory cytokines. Oral enoxaparin significantly reduces the inflammatory pathology associated with DSS-induced colitis in mice and could therefore represent a novel therapeutic option for the management of ulcerative colitis.
Conflict of interest statement
Figures










References
-
- Sands BE, Kaplan GG. The role of TNFα in ulcerative colitis. J Clin Pharmacol. 2007;47: 930–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

