Expression and Subcellular Distribution of GFP-Tagged Human Tetraspanin Proteins in Saccharomyces cerevisiae
- PMID: 26218426
- PMCID: PMC4517926
- DOI: 10.1371/journal.pone.0134041
Expression and Subcellular Distribution of GFP-Tagged Human Tetraspanin Proteins in Saccharomyces cerevisiae
Abstract
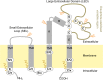
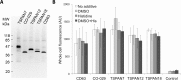
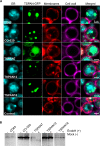
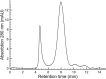
Tetraspanins are integral membrane proteins that function as organizers of multimolecular complexes and modulate function of associated proteins. Mammalian genomes encode approximately 30 different members of this family and remotely related eukaryotic species also contain conserved tetraspanin homologs. Tetraspanins are involved in a number of fundamental processes such as regulation of cell migration, fusion, immunity and signaling. Moreover, they are implied in numerous pathological states including mental disorders, infectious diseases or cancer. Despite the great interest in tetraspanins, the structural and biochemical basis of their activity is still largely unknown. A major bottleneck lies in the difficulty of obtaining stable and homogeneous protein samples in large quantities. Here we report expression screening of 15 members of the human tetraspanin superfamily and successful protocols for the production in S. cerevisiae of a subset of tetraspanins involved in human cancer development. We have demonstrated the subcellular localization of overexpressed tetraspanin-green fluorescent protein fusion proteins in S. cerevisiae and found that despite being mislocalized, the fusion proteins are not degraded. The recombinantly produced tetraspanins are dispersed within the endoplasmic reticulum membranes or localized in granule-like structures in yeast cells. The recombinantly produced tetraspanins can be extracted from the membrane fraction and purified with detergents or the poly (styrene-co-maleic acid) polymer technique for use in further biochemical or biophysical studies.
Conflict of interest statement
Figures


Similar articles
-
Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo- and heterodimers when expressed in yeast.Plant Physiol. 2013 Oct;163(2):696-712. doi: 10.1104/pp.113.216598. Epub 2013 Aug 14. Plant Physiol. 2013. PMID: 23946353 Free PMC article.
-
The tetraspanin network modulates MT1-MMP cell surface trafficking.Int J Biochem Cell Biol. 2013 Jun;45(6):1133-44. doi: 10.1016/j.biocel.2013.02.020. Epub 2013 Mar 14. Int J Biochem Cell Biol. 2013. PMID: 23500527
-
Reducing isoform complexity of human tetraspanins by optimized expression in Dictyostelium discoideum enables high-throughput functional read-out.Protein Expr Purif. 2017 Jul;135:8-15. doi: 10.1016/j.pep.2017.04.012. Epub 2017 Apr 22. Protein Expr Purif. 2017. PMID: 28442431
-
Tetraspanins shape the synapse.Mol Cell Neurosci. 2018 Sep;91:76-81. doi: 10.1016/j.mcn.2018.04.001. Epub 2018 Apr 6. Mol Cell Neurosci. 2018. PMID: 29631019 Review.
-
Tetraspanin genes in plants.Plant Sci. 2012 Jul;190:9-15. doi: 10.1016/j.plantsci.2012.03.005. Epub 2012 Mar 23. Plant Sci. 2012. PMID: 22608515 Review.
Cited by
-
Extraction and liposome reconstitution of membrane proteins with their native lipids without the use of detergents.Sci Rep. 2018 Oct 8;8(1):14950. doi: 10.1038/s41598-018-33208-1. Sci Rep. 2018. PMID: 30297885 Free PMC article.
-
Isolation of yeast complex IV in native lipid nanodiscs.Biochim Biophys Acta. 2016 Dec;1858(12):2984-2992. doi: 10.1016/j.bbamem.2016.09.004. Epub 2016 Sep 13. Biochim Biophys Acta. 2016. PMID: 27620332 Free PMC article.
-
The styrene-maleic acid copolymer: a versatile tool in membrane research.Eur Biophys J. 2016 Jan;45(1):3-21. doi: 10.1007/s00249-015-1093-y. Epub 2015 Dec 6. Eur Biophys J. 2016. PMID: 26639665 Free PMC article. Review.
-
CD81 extracted in SMALP nanodiscs comprises two distinct protein populations within a lipid environment enriched with negatively charged headgroups.Biochim Biophys Acta Biomembr. 2020 Nov 1;1862(11):183419. doi: 10.1016/j.bbamem.2020.183419. Epub 2020 Jul 28. Biochim Biophys Acta Biomembr. 2020. PMID: 32735789 Free PMC article.
-
A new panel of epitope mapped monoclonal antibodies recognising the prototypical tetraspanin CD81.Wellcome Open Res. 2017 Sep 7;2:82. doi: 10.12688/wellcomeopenres.12058.1. eCollection 2017. Wellcome Open Res. 2017. PMID: 29090272 Free PMC article.
References
-
- Boavida LC, Qin P, Broz M, Becker JD, McCormick S. Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo- and heterodimers when expressed in yeast. Plant Physiol. American Society of Plant Biologists; 2013;163: 696–712. 10.1104/pp.113.216598 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

