A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- PMID: 26218535
- PMCID: PMC4517807
- DOI: 10.1371/journal.ppat.1004974
A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
Abstract
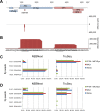
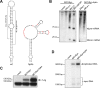
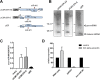
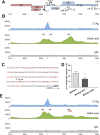
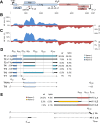
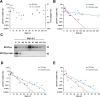
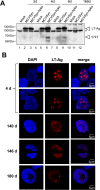
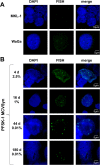
Merkel cell polyomavirus (MCPyV) is considered the etiological agent of Merkel cell carcinoma and persists asymptomatically in the majority of its healthy hosts. Largely due to the lack of appropriate model systems, the mechanisms of viral replication and MCPyV persistence remain poorly understood. Using a semi-permissive replication system, we here report a comprehensive analysis of the role of the MCPyV-encoded microRNA (miRNA) mcv-miR-M1 during short and long-term replication of authentic MCPyV episomes. We demonstrate that cells harboring intact episomes express high levels of the viral miRNA, and that expression of mcv-miR-M1 limits DNA replication. Furthermore, we present RACE, RNA-seq and ChIP-seq studies which allow insight in the viral transcription program and mechanisms of miRNA expression. While our data suggest that mcv-miR-M1 can be expressed from canonical late strand transcripts, we also present evidence for the existence of an independent miRNA promoter that is embedded within early strand coding sequences. We also report that MCPyV genomes can establish episomal persistence in a small number of cells for several months, a time period during which viral DNA as well as LT-Ag and viral miRNA expression can be detected via western blotting, FISH, qPCR and southern blot analyses. Strikingly, despite enhanced replication in short term DNA replication assays, a mutant unable to express the viral miRNA was severely limited in its ability to establish long-term persistence. Our data suggest that MCPyV may have evolved strategies to enter a non- or low level vegetative stage of infection which could aid the virus in establishing and maintaining a lifelong persistence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

