The impairment of learning and memory and synaptic loss in mouse after chronic nitrite exposure
- PMID: 26218639
- PMCID: PMC5516168
- DOI: 10.1002/tox.22174
The impairment of learning and memory and synaptic loss in mouse after chronic nitrite exposure
Abstract
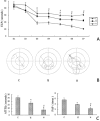
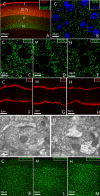
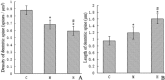
The objective of this study is to understand the impairment of learning and memory in mouse after chronic nitrite exposure. The animal model of nitrite exposure in mouse was created with the daily intubation of nitrite in adult healthy male mice for 3 months. Furthermore, the mouse's learning and memory abilities were tested with Morris water maze, and the expression of Synaptophysin and γ-Synuclein was visualized with immunocytochemistry and Western blot. Our results showed that nitrite exposure significantly prolonged the escape latency period (ELP) and decreased the values of the frequency across platform (FAP) as well as the accumulative time in target quadrant (ATITQ) compared to control, in dose-dependent manner. In addition, after nitrite exposure, synaptophysin (SYN) positive buttons in the visual cortex was reduced, in contrast the increase of γ-synuclein positive cells. The results above were supported by Western blot as well. We conclude that nitrite exposure could lead to a decline in mice's learning and memory. The overexpression of γ-synuclein contributed to the synaptic loss, which is most likely the cause of learning and memory impairment. © 2015 Wiley Periodicals, Inc. Environ Toxicol 31: 1720-1730, 2016.
Keywords: learning and memory; nitrite exposure; synapse; γ-synuclein.
© 2015 Wiley Periodicals, Inc.
Figures


References
-
- Bankiewicz KS, Plunkett RJ, Jacobowitz DM, Porrino L, di Porzio U, London WT, Kopin IJ, Oldfield EH. 1990. The effect of fetal mesencephalon implants on primate MPTP‐induced parkinsonism: Histochemical and behavioral studies. J Neurosurg 72:231–244. - PubMed
-
- Brender JD, Olive JM, Felkner M, Suarez L, Marckwardt W, Hendricks KA. 2004. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology 15:330–336. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

