Heparin and related polysaccharides: synthesis using recombinant enzymes and metabolic engineering
- PMID: 26219501
- PMCID: PMC4546523
- DOI: 10.1007/s00253-015-6821-9
Heparin and related polysaccharides: synthesis using recombinant enzymes and metabolic engineering
Abstract
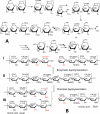
Glycosaminoglycans are linear anionic polysaccharides that exhibit a number of important biological and pharmacological activities. The two most prominent members of this class of polysaccharides are heparin/heparan sulfate and the chondroitin sulfates (including dermatan sulfate). These polysaccharides, having complex structures and polydispersity, are biosynthesized in the Golgi of most animal cells. The chemical synthesis of these glycosaminoglycans is precluded by their structural complexity. Today, we depend on food animal tissues for their isolation and commercial production. Ton quantities of these glycosaminoglycans are used annually as pharmaceuticals and nutraceuticals. The variability of animal-sourced glycosaminoglycans, their inherent impurities, the limited availability of source tissues, the poor control of these source materials, and their manufacturing processes suggest a need for new approaches for their production. Over the past decade, there have been major efforts in the biotechnological production of these glycosaminoglycans. This mini-review focuses on the use of recombinant enzymes and metabolic engineering for the production of heparin and chondroitin sulfates.
Figures




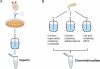
References
-
- Adebowale AO, Cox DS, Liang Z, Eddington ND. Analysis of Glucosamine and Chondroitin Sulfate Content in Marketed Products and the Caco-2 Permeability of Chondroitin Sulfate Raw Materials. 2000;3:37–44.
-
- Bhan N, Xu P, Koffas M a G. Pathway and protein engineering approaches to produce novel and commodity small molecules. Curr Opin Biotechnol. 2013;24:1137–1143. doi: 10.1016/j.copbio.2013.02.019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

