Alphavirus RNA synthesis and non-structural protein functions
- PMID: 26219641
- PMCID: PMC4635493
- DOI: 10.1099/jgv.0.000249
Alphavirus RNA synthesis and non-structural protein functions
Abstract
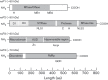
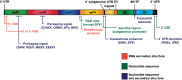
The members of the genus Alphavirus are positive-sense RNA viruses, which are predominantly transmitted to vertebrates by a mosquito vector. Alphavirus disease in humans can be severely debilitating, and depending on the particular viral species, infection may result in encephalitis and possibly death. In recent years, alphaviruses have received significant attention from public health authorities as a consequence of the dramatic emergence of chikungunya virus in the Indian Ocean islands and the Caribbean. Currently, no safe, approved or effective vaccine or antiviral intervention exists for human alphavirus infection. The molecular biology of alphavirus RNA synthesis has been well studied in a few species of the genus and represents a general target for antiviral drug development. This review describes what is currently understood about the regulation of alphavirus RNA synthesis, the roles of the viral non-structural proteins in this process and the functions of cis-acting RNA elements in replication, and points to open questions within the field.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

