Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke
- PMID: 26219646
- PMCID: PMC4542567
- DOI: 10.1161/STROKEAHA.115.009854
Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke
Abstract
Background and purpose: Adult stem cell therapy is an experimental stroke treatment. Here, we assessed homing and anti-inflammatory effects of bone marrow stromal cells (hBMSCs) in chronic stroke.
Methods: At 60 days post stroke, adult Sprague-Dawley rats received intravenous hBMSCs (4×10(6) labeled or nonlabeled cells) or vehicle (saline). A sham surgery group served as additional control. In vivo imaging was conducted between 1 hour and 11 days post transplantation, followed by histological examination.
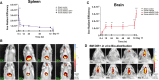
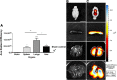
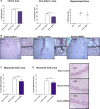
Results: Labeled hBMSCs migrated to spleen which emitted significantly higher fluorescent signal across all time points, especially during the first hour, and were modestly detected in the head region at the 12 hours and 11 days, compared with nonlabeled hBMSCs and vehicle-infused stroke animals, or sham (P<0.05). At 11 days post transplantation, ex vivo imaging confirmed preferential hBMSC migration to the spleen over the brain. Hematoxylin and eosin staining revealed significant 15% and 30% reductions in striatal infarct and peri-infarct area, and a trend of rescue against neuronal loss in the hippocampus. Unbiased stereology showed significant 75% and 60% decrements in major histocompatibility complex II-activated inflammatory cells in gray and white matter, and a 43% diminution in tumor necrosis factor-α cell density in the spleen of transplanted stroke animals compared with vehicle-infused stroke animals (P<0.05). Human antigen immunostaining revealed 0.03% hBMSCs survived in spleen and only 0.0007% in brain. MSC migration to spleen, but not brain, inversely correlated with reduced infarct, peri-infarct, and inflammation.
Conclusions: hBMSC transplantation is therapeutic in chronic stroke possibly by abrogating the inflammation-plagued secondary cell death.
Keywords: brain ischemia; imaging; inflammation; regeneration; stem cells.
© 2015 The Authors.
Figures



References
-
- Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13:171–177. doi: 10.1016/j.jstrokecerebrovasdis.2004.06.003. - PubMed
-
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. - PubMed
-
- The NINDS rt-PA Stroke Study Group. Intracerebral hemorrhage after intravenous tPA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

