Maximizing the Therapeutic Potential of HSP90 Inhibitors
- PMID: 26219697
- PMCID: PMC4645455
- DOI: 10.1158/1541-7786.MCR-15-0234
Maximizing the Therapeutic Potential of HSP90 Inhibitors
Abstract
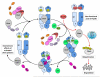
HSP90 is required for maintaining the stability and activity of a diverse group of client proteins, including protein kinases, transcription factors, and steroid hormone receptors involved in cell signaling, proliferation, survival, oncogenesis, and cancer progression. Inhibition of HSP90 alters the HSP90-client protein complex, leading to reduced activity, misfolding, ubiquitination, and, ultimately, proteasomal degradation of client proteins. HSP90 inhibitors have demonstrated significant antitumor activity in a wide variety of preclinical models, with evidence of selectivity for cancer versus normal cells. In the clinic, however, the efficacy of this class of therapeutic agents has been relatively limited to date, with promising responses mainly observed in breast and lung cancer, but no major activity seen in other tumor types. In addition, adverse events and some significant toxicities have been documented. Key to improving these clinical outcomes is a better understanding of the cellular consequences of inhibiting HSP90 that may underlie treatment response or resistance. This review considers the recent progress that has been made in the study of HSP90 and its inhibitors and highlights new opportunities to maximize their therapeutic potential.
©2015 American Association for Cancer Research.
Figures
References
-
- Ellis RJ. Molecular chaperones: assisting assembly in addition to folding. Trends Biochem Sci. 2006;31:395–401. - PubMed
-
- Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–45. - PubMed
-
- Langer T, Fasold H. Isolation and quantification of the heat shock protein 90 alpha and beta isoforms from rat liver. Protoplasma. 2001;218:54–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

