Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line
- PMID: 26219933
- PMCID: PMC4518513
- DOI: 10.1186/s12906-015-0765-6
Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line
Abstract
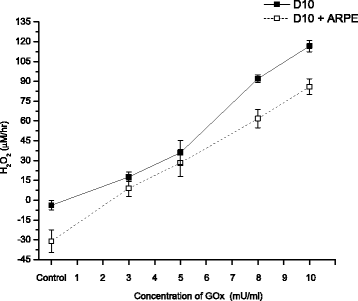
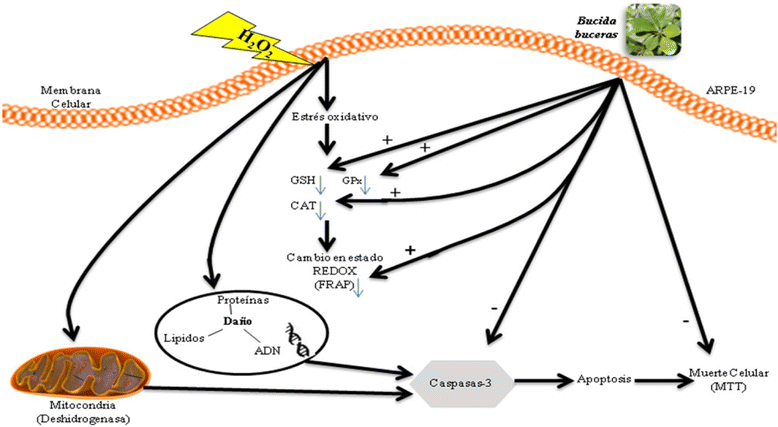
Background: Reactive Oxygen Species (ROS) impair the physiological functions of Retinal Pigment Epithelial (RPE) cells, which are known as one major cause of age-related macular degeneration and retinopathy diseases. The purpose of this study is to explore the cytoprotective effects of the antioxidant Bucida buceras extract in co-treatment with hydrogen peroxide (H2O2) delivery as a single addition or with continuous generation using glucose oxidase (GOx) in ARPE-19 cell cultures. The mechanism of Bucida buceras extract is believed to be associated with their antioxidant capacity to protect cells against oxidative stress.
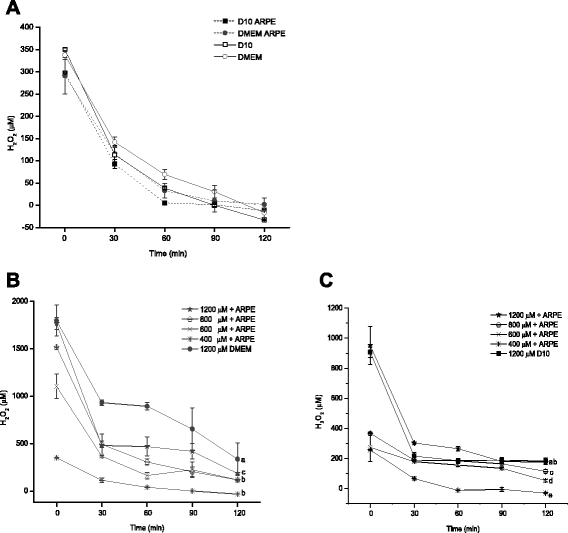
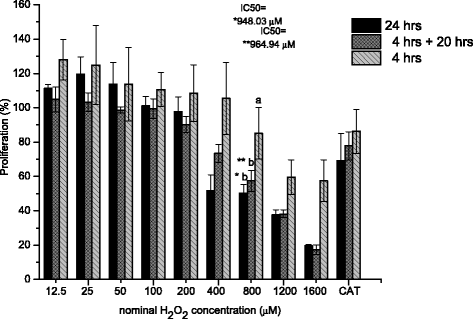
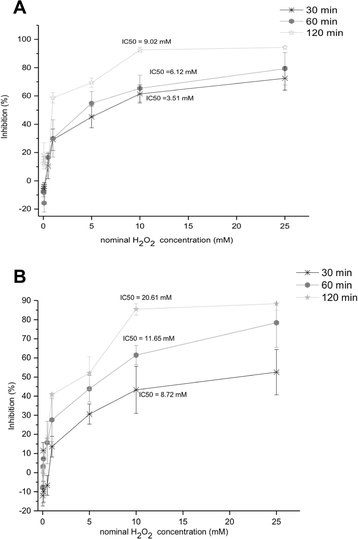
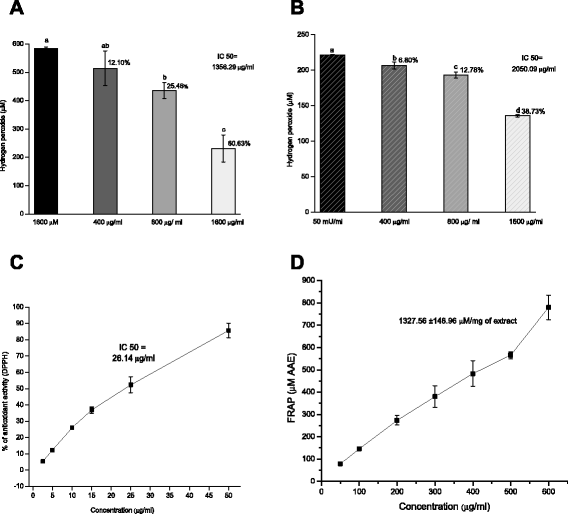
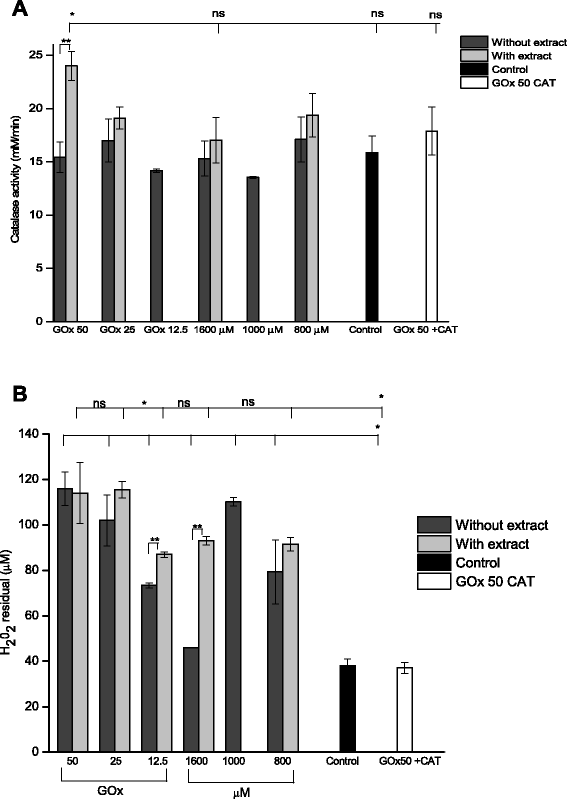
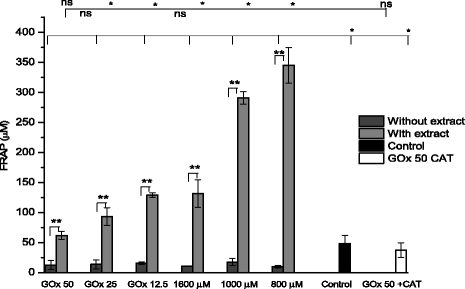
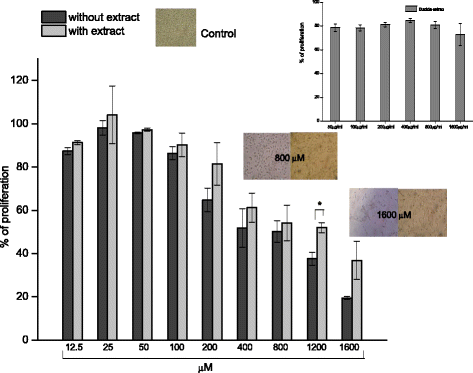
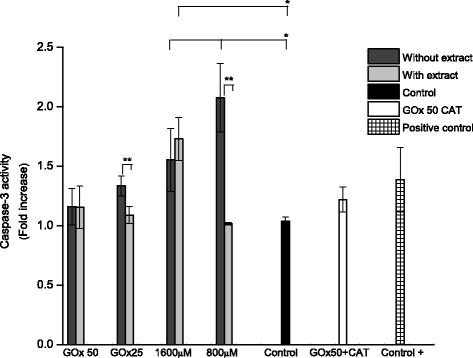
Methods: A comparative oxidative stress H2O2-induced was performed by addition and enzymatic generation using glucose oxidase on human retinal pigment epithelial cells line. H2O2-induced injury was measured by toxic effects (cell death and apoptotic pathway) and intracellular redox status: glutathione (GSH), antioxidant enzymes (catalase and glutathione peroxidase) and reducing power (FRAP). The retino-protective effect of co-treatment with Bucida buceras extract on H2O2-induced human RPE cell injury was investigated by cell death (MTT assay) and oxidative stress biomarkers (H2O2, GSH, CAT, GPx and FRAP).
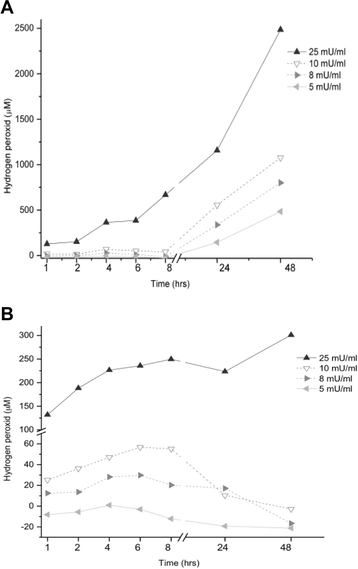
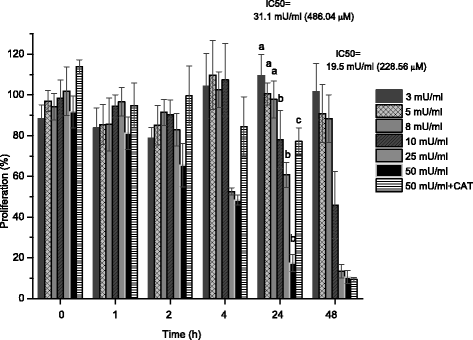
Results: Bucida buceras L. extract is believed to be associated with the ability to prevent cellular oxidative stress. When added as a pulse, H2O2 is rapidly depleted and the cytotoxicity analyses show that cells can tolerate short exposure to high peroxide doses delivered as a pulse but are susceptible to lower chronic doses. Co-treatment with Bucida buceras was able to protect the cells against H2O2-induced injury. In addition to preventing cell death treatment with antioxidant plant could also reverse the significant decrease in GSH level, catalase activity and reducing power caused by H2O2.
Conclusion: These findings suggest that Bucida buceras could protect RPE against ocular pathogenesis associated with oxidative stress induced by H2O2-delivered by addition and enzymatic generation.
Figures





Similar articles
-
Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum.BMC Res Notes. 2015 Sep 1;8:396. doi: 10.1186/s13104-015-1388-1. BMC Res Notes. 2015. PMID: 26323940 Free PMC article.
-
Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells.Int J Mol Sci. 2021 Apr 29;22(9):4722. doi: 10.3390/ijms22094722. Int J Mol Sci. 2021. PMID: 33946898 Free PMC article.
-
Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells.Biosci Rep. 2019 Aug 15;39(8):BSR20190689. doi: 10.1042/BSR20190689. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31345961 Free PMC article.
-
Dissecting in vivo and in vitro redox responses using chemogenetics.Free Radic Biol Med. 2021 Dec;177:360-369. doi: 10.1016/j.freeradbiomed.2021.11.006. Epub 2021 Nov 6. Free Radic Biol Med. 2021. PMID: 34752919 Free PMC article. Review.
-
Peroxiredoxins as Potential Targets for Cardiovascular Disease.Antioxidants (Basel). 2021 Aug 3;10(8):1244. doi: 10.3390/antiox10081244. Antioxidants (Basel). 2021. PMID: 34439492 Free PMC article. Review.
Cited by
-
Neuroprotective effect of apigenin against cerebral ischemia/reperfusion injury.J Int Med Res. 2020 Sep;48(9):300060520945859. doi: 10.1177/0300060520945859. J Int Med Res. 2020. PMID: 32993408 Free PMC article.
-
Ferulic acid (FA) protects human retinal pigment epithelial cells from H2 O2 -induced oxidative injuries.J Cell Mol Med. 2020 Nov;24(22):13454-13462. doi: 10.1111/jcmm.15970. Epub 2020 Oct 20. J Cell Mol Med. 2020. PMID: 33079459 Free PMC article.
-
Retinal Protection of New Nutraceutical Formulation.Pharmaceutics. 2025 Jan 7;17(1):73. doi: 10.3390/pharmaceutics17010073. Pharmaceutics. 2025. PMID: 39861721 Free PMC article.
-
Protein from Hylocereus polyrhizus protects MRC-5 cells against hydrogen peroxide (H2O2)-induced damage.Food Sci Biotechnol. 2022 Oct 12;31(13):1741-1751. doi: 10.1007/s10068-022-01163-3. eCollection 2022 Dec. Food Sci Biotechnol. 2022. PMID: 36312996 Free PMC article.
-
Orexin-A protects SH-SY5Y cells against H2O2-induced oxidative damage via the PI3K/MEK1/2/ERK1/2 signaling pathway.Int J Immunopathol Pharmacol. 2018 Jan-Dec;32:2058738418785739. doi: 10.1177/2058738418785739. Int J Immunopathol Pharmacol. 2018. PMID: 29983082 Free PMC article.
References
-
- Miranda M, Arnal E, Ahuja A, Alvarez R, López R, Ekstrom P, Romero F. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free Radic. Biol. Med. 2010; 48:216–22. - PubMed
-
- Lewis LLM, Iloki ASB, Fernández AD, Gil SAA, Lara ECL, Rubio PJL. Oxidative stress implications for the pathogenesis of ocular pathology. International Journal of Development Research 2015;5(2)3275–288, February.
-
- Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radical Biology and Medicine, 2013;57:176–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

