Sirt1-deficiency causes defective protein quality control
- PMID: 26219988
- PMCID: PMC4518232
- DOI: 10.1038/srep12613
Sirt1-deficiency causes defective protein quality control
Abstract
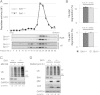
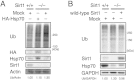
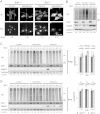
Protein quality control is an important mechanism to maintain cellular homeostasis. Damaged proteins have to be restored or eliminated by degradation, which is mainly achieved by molecular chaperones and the ubiquitin-proteasome system. The NAD(+)-dependent deacetylase Sirt1 has been reported to play positive roles in the regulation of cellular homeostasis in response to various stresses. However, its contribution to protein quality control remains unexplored. Here we show that Sirt1 is involved in protein quality control in both an Hsp70-dependent and an Hsp70-independent manner. Loss of Sirt1 led to the accumulation of ubiquitinated proteins in cells and tissues, especially upon heat stress, without affecting proteasome activities. This was partly due to decreased basal expression of Hsp70. However, this accumulation was only partially alleviated by overexpression of Hsp70 or induction of Hsp70 upon heat shock in Sirt1-deficient cells and tissues. These results suggest that Sirt1 mediates both Hsp70-dependent and Hsp70-independent protein quality control. Our findings cast new light on understanding the role of Sirt1 in maintaining cellular homeostasis.
Figures


References
-
- Boillée S., Vande Velde C. & Cleveland D. W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006). - PubMed
-
- Hartl F. U. & Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002). - PubMed
-
- Schubert U. et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770–774 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

