Tuberous sclerosis complex neuropathology requires glutamate-cysteine ligase
- PMID: 26220190
- PMCID: PMC4518593
- DOI: 10.1186/s40478-015-0225-z
Tuberous sclerosis complex neuropathology requires glutamate-cysteine ligase
Abstract
Introduction: Tuberous sclerosis complex (TSC) is a genetic disease resulting from mutation in TSC1 or TSC2 and subsequent hyperactivation of mammalian Target of Rapamycin (mTOR). Common TSC features include brain lesions, such as cortical tubers and subependymal giant cell astrocytomas (SEGAs). However, the current treatment with mTOR inhibitors has critical limitations. We aimed to identify new targets for TSC pharmacotherapy.
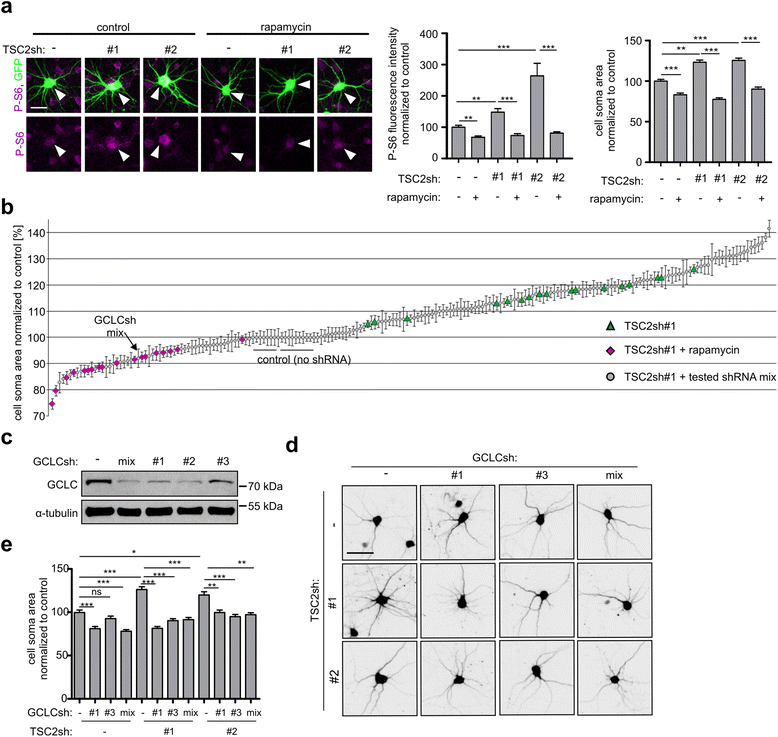
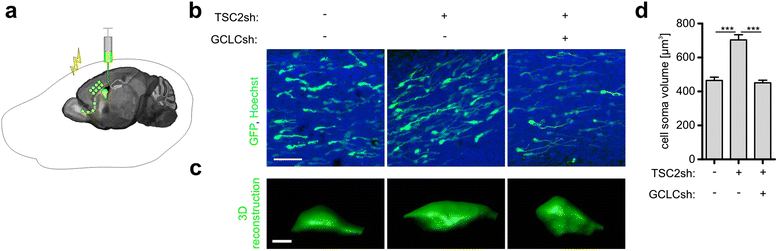
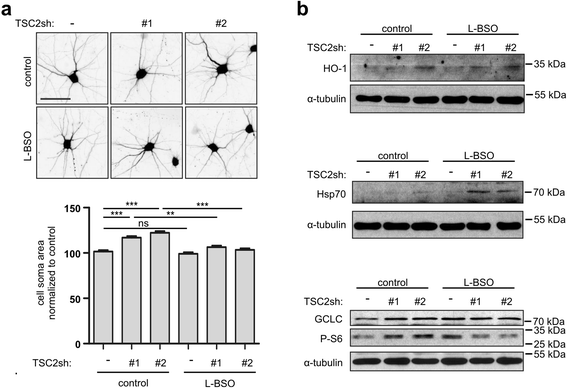
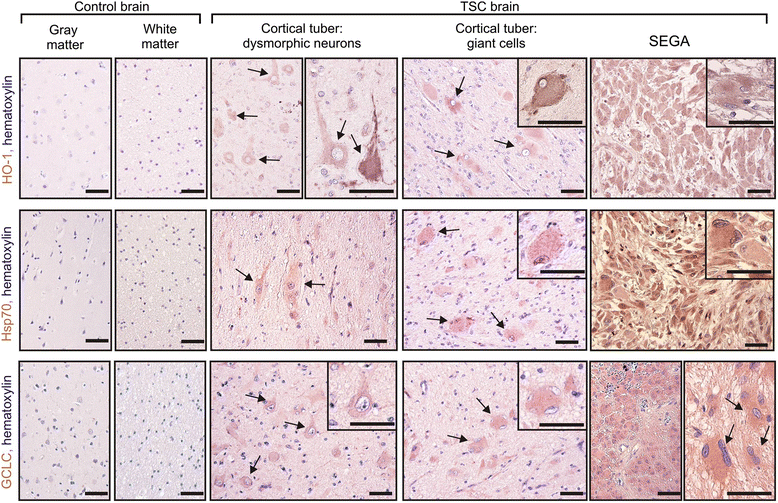
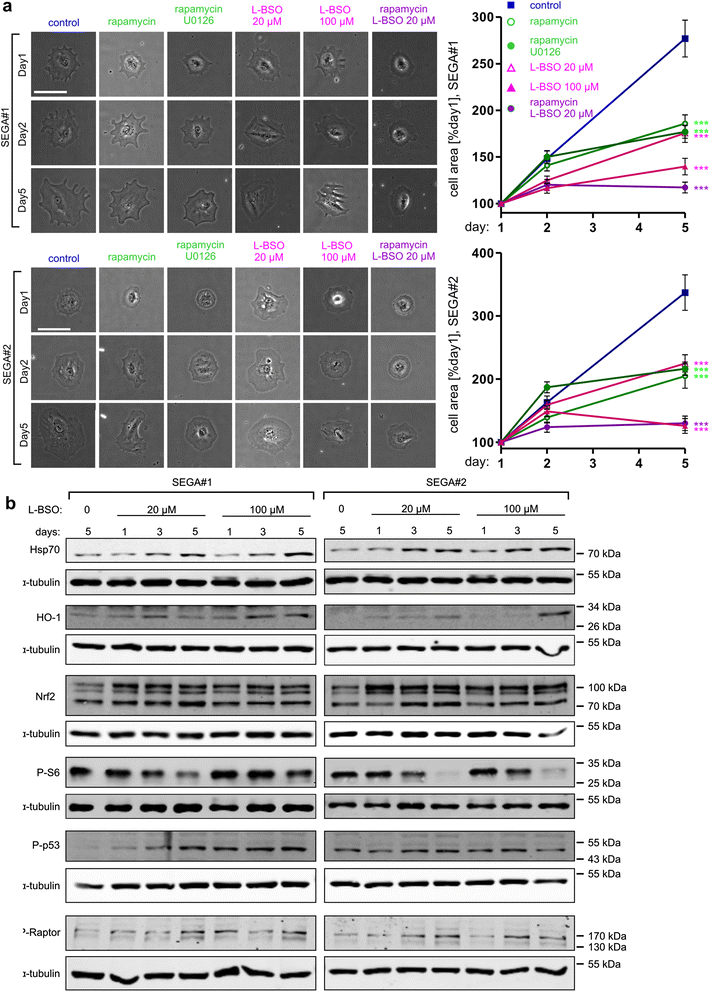
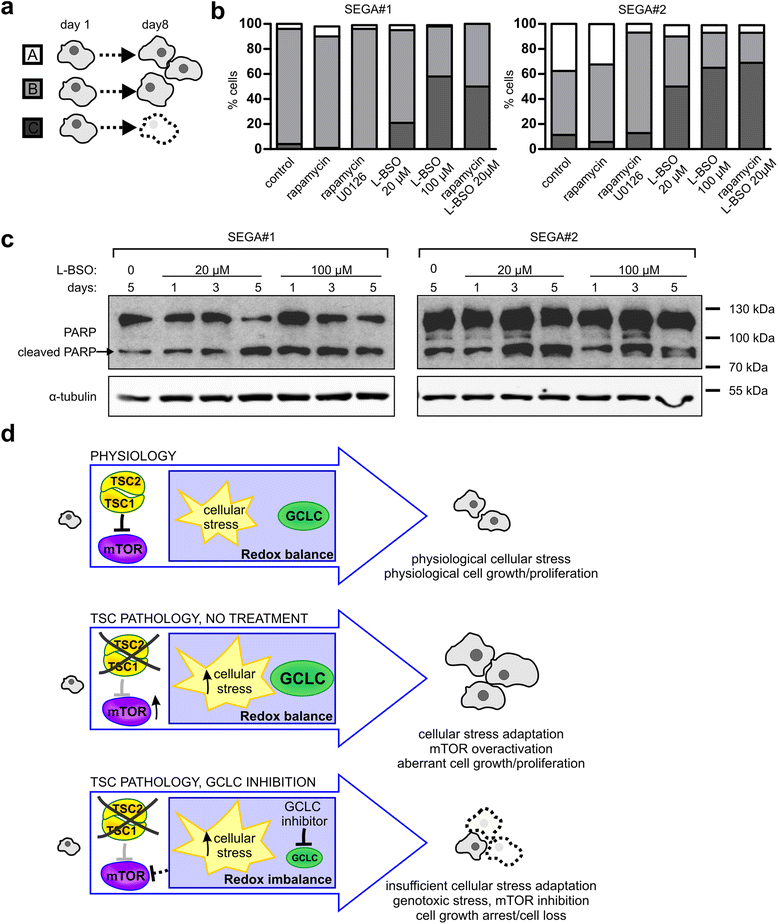
Results: The results of our shRNA screen point to glutamate-cysteine ligase catalytic subunit (GCLC), a key enzyme in glutathione synthesis, as a contributor to TSC-related phenotype. GCLC inhibition increased cellular stress and reduced mTOR hyperactivity in TSC2-depleted neurons and SEGA-derived cells. Moreover, patients' brain tubers showed elevated GCLC and stress markers expression. Finally, GCLC inhibition led to growth arrest and death of SEGA-derived cells.
Conclusions: We describe GCLC as a part of redox adaptation in TSC, needed for overgrowth and survival of mutant cells, and provide a potential novel target for SEGA treatment.
Figures
References
-
- Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol. 1994;12:194–205. - PubMed
-
- Buccoliero AM, Franchi A, Castiglione F, Gheri CF, Mussa F, Giordano F, Genitori L, Taddei GL. Subependymal giant cell astrocytoma (SEGA): Is it an astrocytoma? Morphological, immunohistochemical and ultrastructural study. Neuropathology. 2009;29:25–30. doi: 10.1111/j.1440-1789.2008.00934.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

