Prehospital use of plasma in traumatic hemorrhage (The PUPTH Trial): study protocol for a randomised controlled trial
- PMID: 26220293
- PMCID: PMC4518517
- DOI: 10.1186/s13063-015-0844-5
Prehospital use of plasma in traumatic hemorrhage (The PUPTH Trial): study protocol for a randomised controlled trial
Abstract
Background: Severe traumatic injury and haemorrhagic shock are frequently associated with disruptions of coagulation function (such as trauma-induced coagulopathy TIC) and activation of inflammatory cascades. These pathologies may be exacerbated by current standard of care resuscitation protocols. Observational studies suggest early administration of plasma to severely-injured haemorrhaging patients may correct TIC, minimise inflammation, and improve survival. The proposed randomised clinical trial will evaluate the clinical effectiveness of pre-hospital plasma administration compared with standard- of-care crystalloid resuscitation in severely-injured patients with major traumatic haemorrhage.
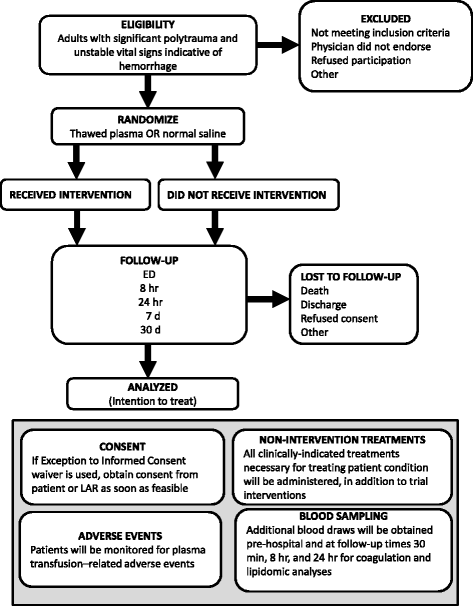
Methods/design: This is a prospective, randomized, open-label, non-blinded trial to determine the effect of pre-hospital administration of thawed plasma (TP) on mortality, morbidity, transfusion requirements, coagulation, and inflammatory response in severely-injured bleeding trauma patients. Two hundred and ten eligible adult trauma patients will be randomised to receive either two units of plasma, to be administered in-field, vs standard of care normal saline (NS). Main analyses will compare subjects allocated to TP to those allocated to NS, on an intention-to-treat basis. Primary outcome measure is all-cause 30-day mortality. Secondary outcome measures include coagulation and lipidomic/pro-inflammatory marker responses, volume of resuscitation fluids (crystalloid, colloid) and blood products administered, and major hospital outcomes (e.g. incidence of MSOF, length of ICU stay, length of hospital stay).
Discussion: This study is part of a US Department of Defense (DoD)-funded multi-institutional investigation, conducted independently of, but in parallel with, the University of Pittsburgh and University of Denver. Demonstration of significant reductions in mortality and coagulopathic/inflammatory-related morbidities as a result of pre-hospital plasma administration would be of considerable clinical importance for the management of haemorrhagic shock in both civilian and military populations.
Trial registration: ClinicalTrials.gov: NCT02303964 on 28 November 2014.
Trial registration: ClinicalTrials.gov NCT01818427 NCT01838863 NCT02303964.
Figures
References
-
- ATLS . Committee on Trauma, American College of Surgeons. Advanced Trauma Life Support Program for Doctors. Chicago: American College of Surgeons; 1997.
-
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. 3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

