The effectiveness and safety of plum-blossom needle therapy for Tourette syndrome: study protocol for a randomized controlled trial
- PMID: 26220439
- PMCID: PMC4517654
- DOI: 10.1186/s13063-015-0873-0
The effectiveness and safety of plum-blossom needle therapy for Tourette syndrome: study protocol for a randomized controlled trial
Abstract
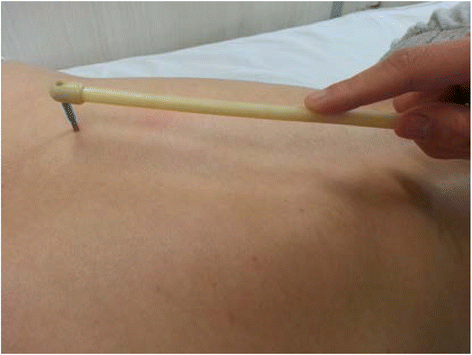
Background: Previous studies have indicated that acupuncture can alleviate the symptoms of Tourette syndrome (TS), but the evidence is insufficient. So far, there have been no reports on plum-blossom needle therapy for TS. Here we present a protocol for a randomized controlled trial using plum-blossom needle therapy to treat TS.
Methods/design: Sixty patients will be randomly allocated into either the plum-blossom needle therapy group or the habit reversal training (HRT) group. All patients in each group will be given 12 weeks of treatment, with follow-up at the 24th week. The primary outcome measure will be the mean change from baseline in the total tic score on the Yale Global Tic Severity Scale (YGTSS) at the 12th week. Secondary outcome measures will include the scores on the TS Clinical Global Impression Scale (CGI) and the mean changes from baseline in the YGTSS score and the Children and Adolescents' Quality of Life Scale (CAQOL) at other time points. Safety will also be evaluated.
Discussion: This trial will evaluate the effectiveness and safety of plum-blossom needle therapy for TS compared with HRT. A limitation of this trial is that patients and acupuncturists cannot be blinded.
Trial registration: ClinicalTrials.gov Identifier: NCT02403258 (Date of registration: March 31, 2015).
Figures



Similar articles
-
Plum-blossom needle therapy for Tourette syndrome: A case report.Explore (NY). 2024 May-Jun;20(3):456-459. doi: 10.1016/j.explore.2023.09.010. Epub 2023 Sep 28. Explore (NY). 2024. PMID: 37783584
-
Sphenopalatine ganglion stimulation with one acupuncture needle for moderate-severe persistent allergic rhinitis: study protocol for a multicenter randomized controlled trial.Trials. 2015 Apr 23;16:183. doi: 10.1186/s13063-015-0707-0. Trials. 2015. PMID: 25899566 Free PMC article. Clinical Trial.
-
Efficacy and safety of using a warming needle for persistent allergic rhinitis: study protocol for a randomized controlled trial.Trials. 2016 Jun 30;17(1):305. doi: 10.1186/s13063-016-1432-z. Trials. 2016. PMID: 27363578 Free PMC article. Clinical Trial.
-
Habit reversal training and educational group treatments for children with tourette syndrome: A preliminary randomised controlled trial.Behav Res Ther. 2016 May;80:43-50. doi: 10.1016/j.brat.2016.03.003. Epub 2016 Mar 23. Behav Res Ther. 2016. PMID: 27037483 Clinical Trial.
-
Treatment of Tourette syndrome by acupuncture combined with Chinese medicine based on syndrome differentiation: A review.Medicine (Baltimore). 2023 Jul 21;102(29):e34268. doi: 10.1097/MD.0000000000034268. Medicine (Baltimore). 2023. PMID: 37478233 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington: American Psychiatric Association; 2000.
-
- Centers for Disease Control and Prevention Prevalence of diagnosed Tourette Syndrome in persons aged 6–17 years - United States, 2007. MMWR Morb and Mortal Wkly Rep. 2009;58(21):581–5. - PubMed
-
- Stern J. Tourette Syndrome. Paediatrics and Child Health. 2014;24(10):447–51. doi: 10.1016/j.paed.2014.03.003. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

