LK6/Mnk2a is a new kinase of alpha synuclein phosphorylation mediating neurodegeneration
- PMID: 26220523
- PMCID: PMC4518213
- DOI: 10.1038/srep12564
LK6/Mnk2a is a new kinase of alpha synuclein phosphorylation mediating neurodegeneration
Abstract
Parkinson's disease (PD) is a movement disorder due to the loss of dopaminergic (DA) neurons in the substantia nigra. Alpha-synuclein phosphorylation and α-synuclein inclusion (Lewy body) become a main contributor, but little is known about their formation mechanism. Here we used protein expression profiling of PD to construct a model of their signalling network from drsophila to human and nominate major nodes that regulate PD development. We found in this network that LK6, a serine/threonine protein kinase, plays a key role in promoting α-synuclein Ser129 phosphorylation by identification of LK6 knockout and overexpression. In vivo test was further confirmed that LK6 indeed enhances α-synuclein phosphorylation, accelerates the death of dopaminergic neurons, reduces the climbing ability and shortens the the life span of drosophila. Further, MAP kinase-interacting kinase 2a (Mnk2a), a human homolog of LK6, also been shown to make α-synuclein phosphorylation and leads to α-synuclein inclusion formation. On the mechanism, the phosphorylation mediated by LK6 and Mnk2a is controlled through ERK signal pathway by phorbolmyristate acetate (PMA) avtivation and PD98059 inhibition. Our findings establish pivotal role of Lk6 and Mnk2a in unprecedented signalling networks, may lead to new therapies preventing α-synuclein inclusion formation and neurodegeneration.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
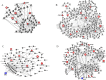
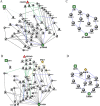
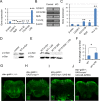
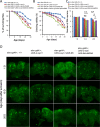
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

