Loss of ATM accelerates pancreatic cancer formation and epithelial-mesenchymal transition
- PMID: 26220524
- PMCID: PMC4532798
- DOI: 10.1038/ncomms8677
Loss of ATM accelerates pancreatic cancer formation and epithelial-mesenchymal transition
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is associated with accumulation of particular oncogenic mutations and recent genetic sequencing studies have identified ataxia telangiectasia-mutated (ATM) mutations in PDAC cohorts. Here we report that conditional deletion of ATM in a mouse model of PDAC induces a greater number of proliferative precursor lesions coupled with a pronounced fibrotic reaction. ATM-targeted mice display altered TGFβ-superfamily signalling and enhanced epithelial-to-mesenchymal transition (EMT) coupled with shortened survival. Notably, our mouse model recapitulates many features of more aggressive human PDAC subtypes. Particularly, we report that low expression of ATM predicts EMT, a gene signature specific for Bmp4 signalling and poor prognosis in human PDAC. Our data suggest an intimate link between ATM expression and pancreatic cancer progression in mice and men.
Figures
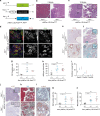
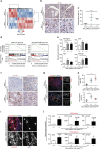
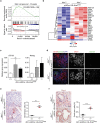
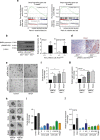



References
-
- Jemal A., Siegel R., Xu J. & Ward E. Cancer statistics, 2010. CA. Cancer J. Clin. 60, 277–300 (2010) . - PubMed
-
- Wellner U. et al.. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495 (2009) . - PubMed
-
- Stathis A. & Moore M. J. Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin.Oncol. 7, 163–172 (2010) . - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

