Addition of a single methyl group to a small molecule sodium channel inhibitor introduces a new mode of gating modulation
- PMID: 26220736
- PMCID: PMC4621986
- DOI: 10.1111/bph.13259
Addition of a single methyl group to a small molecule sodium channel inhibitor introduces a new mode of gating modulation
Retraction in
-
Retracted: Addition of a single methyl group to a small molecule sodium channel inhibitor introduces a new mode of gating modulation, by L Wang, SG Zellmer, DM Printzenhoff and NA Castle. British Journal of Pharmacology, volume 172(20): 4905-4918, published in October 2015; DOI 10.1111/bph.13259.Br J Pharmacol. 2018 Jul;175(13):2712. doi: 10.1111/bph.14349. Epub 2018 May 23. Br J Pharmacol. 2018. PMID: 29906330 Free PMC article.
Abstract
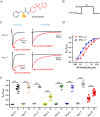
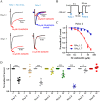
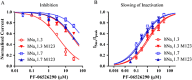
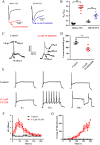
Background and purpose: Aryl sulfonamide Nav 1.3 or Nav 1.7 voltage-gated sodium (Nav ) channel inhibitors interact with the Domain 4 voltage sensor domain (D4 VSD). During studies to better understand the structure-activity relationship of this interaction, an additional mode of channel modulation, specifically slowing of inactivation, was revealed by addition of a single methyl moiety. The objective of the current study was to determine if these different modulatory effects are mediated by the same or distinct interactions with the channel.
Experimental approach: Electrophysiology and site-directed mutation were used to compare the effects of PF-06526290 and its desmethyl analogue PF-05661014 on Nav channel function.
Key results: PF-05661014 selectively inhibits Nav 1.3 versus Nav 1.7 currents by stabilizing inactivated channels via interaction with D4 VSD. In contrast, PF-06526290, which differs from PF-05661014 by a single methyl group, exhibits a dual effect. It greatly slows inactivation of Nav channels in a subtype-independent manner. However, upon prolonged depolarization to induce inactivation, PF-06526290 becomes a Nav subtype selective inhibitor similar to PF-05661014. Mutation of the D4 VSD modulates inhibition of Nav 1.3 or Nav 1.7 by both PF-05661014 and PF-06526290, but has no effect on the inactivation slowing produced by PF-06526290. This finding, along with the absence of functional inhibition of PF-06526290-induced inactivation slowing by PF-05661014, suggests that distinct interactions underlie the two modes of Nav channel modulation.
Conclusions and implications: Addition of a methyl group to a Nav channel inhibitor introduces an additional mode of gating modulation, implying that a single compound can affect sodium channel function in multiple ways.
© 2015 The British Pharmacological Society.
Figures





References
-
- Abbas N, Gaudioso‐Tyzra C, Bonnet C, Gabriac M, Amsalem M, Lonigro A et al. (2013). The scorpion toxin Amm VIII induces pain hypersensitivity through gain‐of‐function of TTX‐sensitive Na(+) channels. Pain 154 (8): 1204–1215. - PubMed
-
- Bagal SK, Chapman ML, Marron BE, Prime R, Storer RI, Swain NA (2014). Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett 24 (16): 3690–3699. - PubMed
-
- Banzawa N, Saito S, Imagawa T, Kashio M, Takahashi K, Tominaga M et al. (2014). Molecular basis determining inhibition/activation of nociceptive receptor TRPA1 protein: a single amino acid dictates species‐specific actions of the most potent mammalian TRPA1 antagonist. J Biol Chem 289 (46): 31927–31939. - PMC - PubMed
-
- Bennett DL, Woods CG (2014). Painful and painless channelopathies. Lancet Neurol 13 (6): 587–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

