Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation
- PMID: 26220754
- PMCID: PMC12182650
- DOI: 10.1016/j.jhep.2015.07.025
Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation
Abstract
Background & aims: In patients with hepatocellular carcinoma (HCC), liver transplantation (LT) is an excellent therapy if tumor characteristics are within the Milan criteria. We aimed to define genomic features enabling to identify HCC patients beyond Milan criteria who have acceptable transplant outcomes.
Methods: Among 770 consecutive HCC patients transplanted between 1990 and 2013, 132 had tumors exceeding Milan criteria on pathology and were enrolled in the study; 44% of the patients satisfied the 'up-to-7 rule' [7=sum of the size of the largest tumor and the number of tumors]. Explant tumors were assessed for genomic signatures and immunohistochemical markers associated with poor outcome.
Results: At a median follow-up of 88months, 64 patients had died and 45 recurred; the 5-year overall survival (OS) and recurrence rates were 57% and 35%, respectively. Cytokeratin 19 (CK19) gene signature was independently associated with recurrence [Hazard ratio (HR)=2.95, p<0.001], along with tumor size (HR=3.37, p=0.023) and presence of satellites (HR=2.98, p=0.001). S2 subclass signature was independently associated with poor OS (HR=3.18, p=0.001), along with tumor size (HR=5.06, p<0.001) and up-to-7 rule (HR=2.50, p=0.002). Using the presence of progenitor cell markers (either CK19 or S2 signatures) patients were classified into poor prognosis (n=58; 5-year recurrence 53%, survival 45%) and good prognosis (n=74; 5-year recurrence 19%, survival 67%) (HR=3.16, p<0.001 for recurrence, and HR=1.72, p=0.04 for OS).
Conclusions: HCC patients transplanted beyond Milan criteria without gene signatures of progenitor markers (CK19 and S2) achieved survival rates similar as those within Milan criteria. Once prospectively validated, these markers may support a limited expansion of LT indications.
Keywords: Gene expression; Gene signature; Prognosis; Stem cell; Survival.
Copyright © 2015 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors who have taken part in this study declared that they do not have any conflict of interest with respect to this manuscript.
Figures

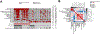
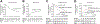
References
-
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–700. - PubMed
-
- Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, et al. J EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Hepatology 2012;56:908–943. - PubMed
-
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–1440. - PubMed
-
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

