Bacterial Cell Enlargement Requires Control of Cell Wall Stiffness Mediated by Peptidoglycan Hydrolases
- PMID: 26220963
- PMCID: PMC4551982
- DOI: 10.1128/mBio.00660-15
Bacterial Cell Enlargement Requires Control of Cell Wall Stiffness Mediated by Peptidoglycan Hydrolases
Abstract
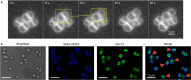
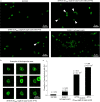
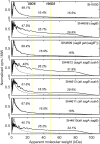
Most bacterial cells are enclosed in a single macromolecule of the cell wall polymer, peptidoglycan, which is required for shape determination and maintenance of viability, while peptidoglycan biosynthesis is an important antibiotic target. It is hypothesized that cellular enlargement requires regional expansion of the cell wall through coordinated insertion and hydrolysis of peptidoglycan. Here, a group of (apparent glucosaminidase) peptidoglycan hydrolases are identified that are together required for cell enlargement and correct cellular morphology of Staphylococcus aureus, demonstrating the overall importance of this enzyme activity. These are Atl, SagA, ScaH, and SagB. The major advance here is the explanation of the observed morphological defects in terms of the mechanical and biochemical properties of peptidoglycan. It was shown that cells lacking groups of these hydrolases have increased surface stiffness and, in the absence of SagB, substantially increased glycan chain length. This indicates that, beyond their established roles (for example in cell separation), some hydrolases enable cellular enlargement by making peptidoglycan easier to stretch, providing the first direct evidence demonstrating that cellular enlargement occurs via modulation of the mechanical properties of peptidoglycan.
Importance: Understanding bacterial growth and division is a fundamental problem, and knowledge in this area underlies the treatment of many infectious diseases. Almost all bacteria are surrounded by a macromolecule of peptidoglycan that encloses the cell and maintains shape, and bacterial cells must increase the size of this molecule in order to enlarge themselves. This requires not only the insertion of new peptidoglycan monomers, a process targeted by antibiotics, including penicillin, but also breakage of existing bonds, a potentially hazardous activity for the cell. Using Staphylococcus aureus, we have identified a set of enzymes that are critical for cellular enlargement. We show that these enzymes are required for normal growth and define the mechanism through which cellular enlargement is accomplished, i.e., by breaking bonds in the peptidoglycan, which reduces the stiffness of the cell wall, enabling it to stretch and expand, a process that is likely to be fundamental to many bacteria.
Copyright © 2015 Wheeler et al.
Figures


Similar articles
-
SagB Glucosaminidase Is a Determinant of Staphylococcus aureus Glycan Chain Length, Antibiotic Susceptibility, and Protein Secretion.J Bacteriol. 2016 Jan 25;198(7):1123-36. doi: 10.1128/JB.00983-15. J Bacteriol. 2016. PMID: 26811319 Free PMC article.
-
Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction.PLoS Pathog. 2021 Mar 31;17(3):e1009468. doi: 10.1371/journal.ppat.1009468. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33788901 Free PMC article.
-
Demonstration of the role of cell wall homeostasis in Staphylococcus aureus growth and the action of bactericidal antibiotics.Proc Natl Acad Sci U S A. 2021 Nov 2;118(44):e2106022118. doi: 10.1073/pnas.2106022118. Proc Natl Acad Sci U S A. 2021. PMID: 34716264 Free PMC article.
-
Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes.J Biol Chem. 2020 Mar 6;295(10):3347-3361. doi: 10.1074/jbc.REV119.010155. Epub 2020 Jan 23. J Biol Chem. 2020. PMID: 31974163 Free PMC article. Review.
-
Staphylococcus aureus cell wall maintenance - the multifaceted roles of peptidoglycan hydrolases in bacterial growth, fitness, and virulence.FEMS Microbiol Rev. 2022 Oct 28;46(5):fuac025. doi: 10.1093/femsre/fuac025. FEMS Microbiol Rev. 2022. PMID: 35675307 Free PMC article. Review.
Cited by
-
Comparative Genomics Identifies Novel Genetic Changes Associated with Oxacillin, Vancomycin and Daptomycin Susceptibility in ST100 Methicillin-Resistant Staphylococcus aureus.Antibiotics (Basel). 2023 Feb 11;12(2):372. doi: 10.3390/antibiotics12020372. Antibiotics (Basel). 2023. PMID: 36830286 Free PMC article.
-
Bacterial Swarming Reduces Proteus mirabilis and Vibrio parahaemolyticus Cell Stiffness and Increases β-Lactam Susceptibility.mBio. 2019 Oct 8;10(5):e00210-19. doi: 10.1128/mBio.00210-19. mBio. 2019. PMID: 31594808 Free PMC article.
-
Is Longitudinal Division in Rod-Shaped Bacteria a Matter of Swapping Axis?Front Microbiol. 2018 May 8;9:822. doi: 10.3389/fmicb.2018.00822. eCollection 2018. Front Microbiol. 2018. PMID: 29867786 Free PMC article. Review.
-
Diamide Inhibitors of the Bacillus subtilis N-Acetylglucosaminidase LytG That Exhibit Antibacterial Activity.ACS Infect Dis. 2017 Jun 9;3(6):421-427. doi: 10.1021/acsinfecdis.7b00005. Epub 2017 May 8. ACS Infect Dis. 2017. PMID: 28448118 Free PMC article.
-
The architecture of the Gram-positive bacterial cell wall.Nature. 2020 Jun;582(7811):294-297. doi: 10.1038/s41586-020-2236-6. Epub 2020 Apr 29. Nature. 2020. PMID: 32523118 Free PMC article.
References
-
- Höltje J-V. 1993. Three for one —a simple growth mechanism that guarantees a precise copy of the thin, rod-shaped murein sacculus of Escherichia coli, p 419–426. In de Pedro MA, Höltje J-V, Löffelhardt W (ed), Bacterial growth and lysis: metabolism and structure of the bacterial sacculus. Springer, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
