Fibroblast growth factor signaling in skeletal development and disease
- PMID: 26220993
- PMCID: PMC4526732
- DOI: 10.1101/gad.266551.115
Fibroblast growth factor signaling in skeletal development and disease
Abstract
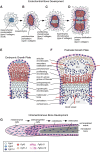
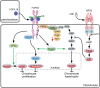
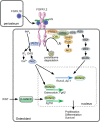
Fibroblast growth factor (FGF) signaling pathways are essential regulators of vertebrate skeletal development. FGF signaling regulates development of the limb bud and formation of the mesenchymal condensation and has key roles in regulating chondrogenesis, osteogenesis, and bone and mineral homeostasis. This review updates our review on FGFs in skeletal development published in Genes & Development in 2002, examines progress made on understanding the functions of the FGF signaling pathway during critical stages of skeletogenesis, and explores the mechanisms by which mutations in FGF signaling molecules cause skeletal malformations in humans. Links between FGF signaling pathways and other interacting pathways that are critical for skeletal development and could be exploited to treat genetic diseases and repair bone are also explored.
Keywords: FGF; FGFR; bone; cartilage; chondrocyte; osteoblast.
© 2015 Ornitz and Marie; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. 2002. The role of the resting zone in growth plate chondrogenesis. Endocrinology 143: 1851–1857. - PubMed
-
- Agas D, Marchetti L, Menghi G, Materazzi S, Materazzi G, Capacchietti M, Hurley MM, Sabbieti MG. 2008. Anti-apoptotic Bcl-2 enhancing requires FGF-2/FGF receptor 1 binding in mouse osteoblasts. J Cell Physiol 214: 145–152. - PubMed
-
- Agas D, Sabbieti MG, Marchetti L, Xiao L, Hurley MM. 2013. FGF-2 enhances Runx-2/Smads nuclear localization in BMP-2 canonical signaling in osteoblasts. J Cell Physiol 228: 2149–2158. - PubMed
-
- Aikawa T, Segre GV, Lee K. 2001. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E–Cdk2. J Biol Chem 276: 29347–29352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
