Microfluidic reflow pumps
- PMID: 26221199
- PMCID: PMC4499048
- DOI: 10.1063/1.4926583
Microfluidic reflow pumps
Abstract
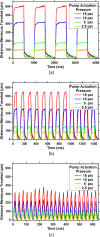
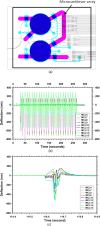
A new microfluidic pump, termed a reflow pump, is designed to operate with a sub-μl sample volume and transport it back and forth between two pneumatically actuated reservoirs through a flow channel typically containing one or more sensor surfaces. The ultimate motivation is to efficiently use the small sample volume in conjunction with convection to maximize analyte flux to the sensor surface(s) in order to minimize sensor response time. In this paper, we focus on the operational properties of the pumps themselves (rather than the sensor surfaces), and demonstrate both two-layer and three-layer polydimethylsiloxane reflow pumps. For the three-layer pump, we examine the effects of reservoir actuation pressure and actuation period, and demonstrate average volumetric flow rates as high as 500 μl/min. We also show that the two-layer design can pump up to 93% of the sample volume during each half period and demonstrate integration of a reflow pump with a single-chip microcantilever array to measure maximum flow rate.
Figures






References
-
- Jeong O. C. and Konishi S., “Fabrication and drive test of pneumatic PDMS micro pump,” Sens. Actuators, A 135, 849–856 (2007).10.1016/j.sna.2006.09.012 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
