Interventions for pain with intrauterine device insertion
- PMID: 26222246
- PMCID: PMC9580985
- DOI: 10.1002/14651858.CD007373.pub3
Interventions for pain with intrauterine device insertion
Abstract
Background: Fear of pain during insertion of intrauterine contraception (IUC) is a barrier to use of this method. IUC includes copper-containing intrauterine devices and levonorgestrel-releasing intrauterine systems. Interventions for pain control during IUC insertion include non-steroidal anti-inflammatory drugs (NSAIDs), local cervical anesthetics, and cervical ripening agents such as misoprostol.
Objectives: To review randomized controlled trials (RCTs) of interventions for reducing IUC insertion-related pain
Search methods: We searched for trials in CENTRAL, MEDLINE, EMBASE, POPLINE, ClinicalTrials.gov, and ICTRP. The most recent search was 22 June 2015. We examined reference lists of pertinent articles. For the initial review, we wrote to investigators to find other published or unpublished trials.
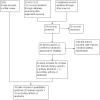
Selection criteria: We included RCTs that evaluated an intervention for preventing IUC insertion-related pain. The comparison could have been a placebo, no intervention, or another active intervention. The primary outcomes were self-reported pain at tenaculum placement, during IUC insertion, and after IUC insertion (up to six hours).
Data collection and analysis: Two authors extracted data from eligible trials. For dichotomous variables, we calculated the Mantel-Haenszel odds ratio (OR) with 95% confidence interval (CI). For continuous variables, we computed the mean difference (MD) with 95% CI. In meta-analysis of trials with different measurement scales, we used the standardized mean difference (SMD).
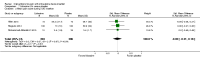
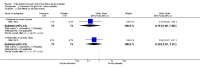
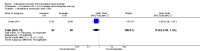
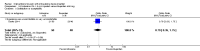
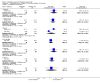
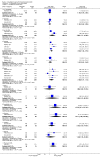
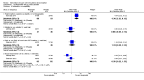
Main results: We included 33 trials with 5710 participants total; 29 were published from 2010 to 2015. Studies examined lidocaine, misoprostol, NSAIDs, and other interventions. Here we synthesize results from trials with sufficient outcome data and moderate- or high-quality evidence.For lidocaine, meta-analysis showed topical 2% gel had no effect on pain at tenaculum placement (two trials) or on pain during IUC insertion (three trials). Other formulations were effective compared with placebo in individual trials. Mean score for IUC-insertion pain was lower with lidocaine and prilocaine cream (MD -1.96, 95% CI -3.00 to -0.92). Among nulliparous women, topical 4% formulation showed lower scores for IUC-insertion pain assessed within 10 minutes (MD -15.90, 95% CI -22.77 to -9.03) and at 30 minutes later (MD -11.10, 95% CI -19.05 to -3.15). Among parous women, IUC-insertion pain was lower with 10% spray (median 1.00 versus 3.00). Compared with no intervention, pain at tenaculum placement was lower with 1% paracervical block (median 12 versus 28).For misoprostol, meta-analysis showed a higher mean score for IUC insertion compared with placebo (SMD 0.27, 95% CI 0.07 to 0.46; four studies). In meta-analysis, cramping was more likely with misoprostol (OR 2.64, 95% CI 1.46 to 4.76; four studies). A trial with nulliparous women found a higher score for IUC-insertion pain with misoprostol (median 46 versus 34). Pain before leaving the clinic was higher for misoprostol in two trials with nulliparous women (MD 7.60, 95% CI 6.48 to 8.72; medians 35.5 versus 20.5). In one trial with nulliparous women, moderate or severe pain at IUC insertion was less likely with misoprostol (OR 0.30, 95% CI 0.16 to 0.55). In the same trial, the misoprostol group was more likely to rate the experience favorably. Within two trials of misoprostol plus diclofenac, shivering, headache, or abdominal pain were more likely with misoprostol. Participants had no vaginal delivery. One trial showed the misoprostol group less likely to choose or recommend the treatment.Among multiparous women, mean score for IUC-insertion pain was lower for tramadol 50 mg versus naproxen 550 mg (MD -0.63, 95% CI -0.94 to -0.32) and for naproxen versus placebo (MD -1.94, 95% CI -2.35 to -1.53). The naproxen group was less likely than the placebo group to report the insertion experience as unpleasant and not want the medication in the future. An older trial showed repeated doses of naproxen 300 mg led to lower pain scores at one hour (MD -1.04, 95% CI -1.67 to -0.41) and two hours (MD -0.98, 95% CI -1.64 to -0.32) after insertion. Most women were nulliparous and also had lidocaine paracervical block.
Authors' conclusions: Nearly all trials used modern IUC. Most effectiveness evidence was of moderate quality, having come from single trials. Lidocaine 2% gel, misoprostol, and most NSAIDs did not help reduce pain. Some lidocaine formulations, tramadol, and naproxen had some effect on reducing IUC insertion-related pain in specific groups. The ineffective interventions do not need further research.
Conflict of interest statement
LM Lopez, A Bernholc, Y Zeng are employed at FHI 360, where Hubacher 2006 was conducted. They were not involved in that trial.
RH Allen has served as a clinical trainer for Merck and as a consultant for Actavis and Bayer. She was an investigator of Allen 2013, an included trial.
D Hubacher has served on Advisory Boards for Bayer HealthCare Pharmaceuticals, Inc., Teva Pharmaceuticals, and OCON Medical. He has received product donation from Bayer HealthCare Pharmaceuticals, Inc., Teva Pharmaceuticals, and Merck Sharp & Dohme Corp. He has received research funding from Bayer HealthCare Pharmaceuticals, Inc. and Duramed. He was an investigator of Hubacher 2006, an included trial.
D Bartz and PA O'Brien have no known conflicts of interest.
Figures







































Update of
-
Interventions for pain with intrauterine device insertion.Cochrane Database Syst Rev. 2009 Jul 8;(3):CD007373. doi: 10.1002/14651858.CD007373.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2015 Jul 29;(7):CD007373. doi: 10.1002/14651858.CD007373.pub3. PMID: 19588429 Updated.
References
References to studies included in this review
Ahmadi Doulabi 2013 {published data only}
-
- Ahmadi Doulabi M, Tavakolian S, Mortazavi A, Akbarzade Baghban A. Effect of EMLA cream on pain of Copper IUD insertion [article in Persian]. Scientific Journal of Hamadan Nursing & Midwifery Faculty 2013;21(3):24‐30.
-
- Ahmadi M. A comparison of EMLA cream and placebo on pain during IUD insertion in women referred to health centers of Imam Hussein. www.irct.ir/searchresult.php?id=5698&number=4 (accessed 23 September 2014). [IRCT201209015698N4]
Aksoy 2015 {published data only}
-
- Acmaz G. Lidocaine 10% spray reduces pain during intrauterine device insertion. clinicaltrials.gov/ct2/show/NCT02020551 (accessed 11 September 2014).
-
- Aksoy H, Aksoy Ü, Ozyurt S, Açmaz G, Babayigit M. Lidocaine 10% spray to the cervix reduces pain during intrauterine device insertion: a double‐blind randomised controlled trial. Journal of Family Planning and Reproductive Health Care 2015 Mar 10 [Epub ahead of print]:1‐5. - PubMed
Allen 2013 {published data only}
-
- Allen RH, Raker C, Goyal V. Higher dose cervical 2% lidocaine gel for IUD insertion: A randomized controlled trial. Contraception 2013; Vol. 88, issue 6:730‐6. - PubMed
Bednarek 2013 {published data only}
-
- Bednarek PH, Micks EA, Edelman AB, Li H, Jensen JT. The effect of nitroprusside on IUD insertion experience in nulliparous women: A pilot study. Contraception 2013;87(4):421‐5. - PubMed
Bednarek 2015 {published data only}
-
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. New England Journal of Medicine 2011;364(23):2208‐17. - PubMed
-
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT, Post‐Aspiration IUD Randomization (PAIR) Study Trial Group. Prophylactic ibuprofen does not improve pain with IUD insertion: a randomized trial. Contraception 2015;91(3):193‐7. - PubMed
-
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Mayo J, et al. Pain with IUD insertion following prophylactic ibuprofen: A randomized trial (abstract). Contraception 2010;82(2):194‐5. - PubMed
Cameron 2013 {published data only}
-
- Cameron ST, Glasier A, Cooper A, Johnstone A. Does a full bladder assist insertion of intrauterine contraception?: a randomised trial. Journal of Family Planning and Reproductive Health Care 2013;39(3):207‐10. - PubMed
Castro 2014 {published data only}
-
- Castro TV, Franceschini SA, Poli‐Neto O, Ferriani RA, Silva de Sa MF, Vieira CS. Effect of intracervical anesthesia on pain associated with the insertion of the levonorgestrel‐releasing intrauterine system in women without previous vaginal delivery: a RCT. Human Reproduction 2014;29(11):2439‐45. - PubMed
-
- Vitti T, Ferriani R, Vieira C. Local anaesthetic in uterine cervix is not associated with lower pain score in levonorgestrel‐releasing intrauterine system insertion in women without previous vaginal delivery (abstract). European Journal of Contraception and Reproductive Health Care 2012;17(S1):S104.
Chor 2012 {published data only}
-
- Chor J, Bregand‐White J, Golobof A, Harwood B, Cowett A. Ibuprofen prophylaxis for levonorgestrel‐releasing intrauterine system insertion: A randomized controlled trial. Contraception 2012;85(6):558‐62. - PubMed
-
- Chor J, Harwood B, Cowett A. Ibuprofen prophylaxis for levonorgestrel‐releasing intrauterine system insertion: A randomized controlled trial (abstract). Contraception 2010;82(2):201‐2. - PubMed
Cırık 2013 {published data only}
-
- Cırık DA, Taşkın EF, Tuğlu A, Ortaç AS, Dai O. Paracervical block with 1% lidocaine for pain control during intrauterine device insertion: a prospective, single‐blinded, controlled study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(3):263‐7.
Dijkhuizen 2011 {published and unpublished data}
-
- Dijkhuizen K, Dekkers OM, Holleboom CA, Groot CJ, Hellebrekers BW, Roosmalen GJ, et al. Vaginal misoprostol prior to insertion of an intrauterine device: an RCT. Human Reproduction 2011;26(2):323‐9. - PubMed
Edelman 2011 {published data only}
-
- Edelman AB, Schaefer E, Olson A, Houten L, Bednarek P, Leclair C, et al. Effects of prophylactic misoprostol administration prior to intrauterine device insertion in nulliparous women. Contraception 2011;84(3):234‐9. - PubMed
Espey 2014 {published data only}
-
- Espey E, Leeman L, Greene H, Singh R, Ogburn T, Fowler K. Misoprostol for IUD insertion in nulliparous women: A randomized controlled trial (abstract). Contraception 2013;88(3):456‐7. - PubMed
-
- Espey E, Singh RH, Leeman L, Ogburn T, Fowler K, Greene H. Misoprostol for intrauterine device insertion in nulliparous women: A randomized controlled trial. American Journal of Obstetrics and Gynecology 2014;210(3):208.e1‐5. - PubMed
Heikinheimo 2010 {published data only}
-
- Gemzell‐Danielsson K, Inki P, Boubli L, O'Flynn M, Kunz M, Heikinheimo O. Bleeding pattern and safety of consecutive use of the levonorgestrel‐releasing intrauterine system (LNG‐IUS)‐‐a multicentre prospective study. Human Reproduction 2010;25(2):354‐9. - PubMed
-
- Heikinheimo O, Inki P, Kunz M, Parmhed S, Anttila AM, Olsson SE, et al. Double‐blind, randomized, placebo‐controlled study on the effect of misoprostol on ease of consecutive insertion of the levonorgestrel‐releasing intrauterine system. Contraception 2010;81(6):481‐6. - PubMed
Hubacher 2006 {published data only}
-
- Hubacher D, Reyes V, Lillo S, Zepeda A, Chen P, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. American Journal of Obstetrics and Gynecology 2006;195(5):1272‐7. - PubMed
Ibrahim 2013 {published data only}
-
- Ibrahim ZM, Sayed Ahmed WA. Sublingual misoprostol prior to insertion of a T380A intrauterine device in women with no previous vaginal delivery. European Journal of Contraception and Reproductive Health Care 2013;18(4):300‐8. - PubMed
Jensen 1998 {published and unpublished data}
-
- Jensen HH, Blaabjerg J, Lyndrup J. Prophylactic use of prostaglandin synthesis inhibitors in connection with IUD insertion. Ugeskrift for Laeger 1998;160(48):6958‐61. - PubMed
Karabayirli 2012 {published data only}
-
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. Journal of Minimally Invasive Gynecology 2012;19(5):581‐4. - PubMed
Lathrop 2013 {published data only}
-
- Lathrop E, Haddad L, McWhorter CP, Goedken P. Self‐administration of misoprostol prior to intrauterine device insertion among nulliparous women: a randomized controlled trial. Contraception 2013;88(6):725‐9. - PubMed
Lotke 2013 {published data only}
-
- Lotke P. Use of misoprostol for intrauterine device (IUD) insertion in nulliparous women. clinicaltrials.gov/show/NCT01001897 (accessed 28 October 2014).
-
- Lotke PS, Tiwari A, Nuño VL. Inserting intrauterine devices in nulliparous women: is misoprostol beneficial? A registered clinical trial. American Journal of Clinical and Experimental Obstetrics Gynecology 2013;1(1):62‐8.
Maguire 2012 {published data only}
-
- Maguire K, Davis A, Rosario Tejeda L, Westhoff C. Intracervical lidocaine gel for intrauterine device insertion: a randomized controlled trial. Contraception 2012;86(3):214‐9. - PubMed
-
- Maguire K, Davis A, Rosario Tejeda L, Westhoff CL. Intracervical 2% lidocaine gel as an analgesic during intrauterine device insertion: A randomized controlled trial (abstract). Contraception 2012;85(3):324. - PubMed
-
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers' assessment of pain during intrauterine device insertion. Contraception 2014;89(1):22‐4. - PubMed
Massey 1974 {published data only}
-
- Massey SE, Varady JC, Henzl MR. Pain relief with naproxen following insertion of an intrauterine device. Journal of Reproductive Medicine 1974;13(6):226‐31. - PubMed
McNicholas 2012 {published data only}
Micks 2014 {published data only}
-
- Micks EA, Jensen JT, Bednarek PH. The effect of nitroglycerin on the IUD insertion experience in nulliparous women: a pilot study. Contraception 2014;90(1):60‐5. - PubMed
Mody 2012 {published data only}
-
- Krishnan S, Kiley J, Rademaker A, Gawron L, Stika C, Hammond C. Pain control for intrauterine device insertion: A randomized trial of 1% lidocaine paracervical block (abstract). Contraception 2011;84(3):319. - PubMed
-
- Mody SK, Kiley J, Rademaker A, Gawron L, Stika C, Hammond C. Pain control for intrauterine device insertion: A randomized trial of 1% lidocaine paracervical block. Contraception 2012;86(6):704‐9. - PubMed
Mohammad‐Alizadeh‐C 2010 {published data only}
-
- Mohammad‐Alizadeh‐Charandabi S, Seidi S, Kazemi F. Effect of lidocaine gel on pain from copper IUD insertion: a randomized double‐blind controlled trial. Indian Journal of Medical Sciences 2010;64(8):349‐55. - PubMed
Nelson 2013 {published data only}
-
- Nelson AL, Fong JK. Intrauterine infusion of lidocaine does not reduce pain scores during IUD insertion. Contraception 2013;88(1):37‐40. - PubMed
Ngo 2014 {published data only}
-
- Mody S. Pain control for intrauterine device placement: a Ttial of ketorolac prior to intrauterine device placement. clinicaltrials.gov/show/NCT01664559 (accessed 11 September 2014). [NCT01664559]
-
- Ngo L, Ward K, Mody S. Pain control for intrauterine device insertion: a randomized double‐blind controlled trial of ketorolac (abstract). Contraception 2014;90(3):297.
Rapkin 2014 {published and unpublished data}
-
- Rapkin RB. Self‐administered intravaginal 2% lidocaine gel prior to intrauterine device insertion in nulliparous women (LIVIIN). clinicaltrials.gov/show/NCT01534520 (accessed 4 September 2014).
-
- Rapkin RB, Achilles SL, Boraas C, Cremer M, Schwarz EB, Chen BA. Self‐administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial (abstract). Obstetrics and Gynecology 2014;123(5 (Suppl)):110S. [NCT01534520] - PubMed
Sääv 2007 {published data only}
-
- Sääv I, Aronsson A, Marions L, Stephansson O, Gemzell‐Danielsson K. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Human Reproduction 2007;22(10):2647‐52. - PubMed
Scavuzzi 2013 {published data only}
-
- Scavuzzi A, Souza AS, Costa AA, Amorim MM. Misoprostol prior to inserting an intrauterine device in nulligravidas: a randomized clinical trial. Human Reproduction 2013;28(8):2118‐25. - PubMed
Shahnazi 2012 {published data only}
Swenson 2012 {published data only}
-
- Swenson C, Turok DK, Ward K, Jacobson JC, Dermish A. Self‐administered misoprostol or placebo before intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstetrics and Gynecology 2012;120(2 Part 1):341‐7. - PubMed
Tornblom‐Paulander 2015 {published and unpublished data}
-
- Pharmanest AB 2013. A randomized, double blind, placebo controlled, parallel group clinical study investigating the analgesic effect and safety of a topical formulation of lidocaine (SHACT) during and after insertion of an Intra Uterine Device. A phase II study. www.clinicaltrialsregister.eu/ctr‐search/trial/2011‐006220‐20/SE (accessed 22 January 2015).
-
- Tingaker B, Brodin A, Irestedt L, Ekman‐Ordeberg G. A novel mucosal pain relief drug candidate ‐ SHACT ‐‐ gives highly significant analgesia at insertion of intra uterine device (IUD) (abstract). Reproductive Sciences 2014;21(3):318A.
-
- Tornblom‐Paulander S, Tingåker BK, Werner A, Liliecreutz C, Conner P, Wessel H, et al. Novel topical formulation of lidocaine provides significant pain relief for intrauterine device insertion: pharmacokinetic evaluation and randomized placebo‐controlled trial. Fertility and Sterility 2015;103(2):422‐7. - PubMed
References to studies excluded from this review
Goldstuck 1983 {published data only}
-
- Goldstuck ND. Treatment of pain following IUD insertion with meptazinol‐a new centrally acting analgesic. Contraceptive Delivery Systems: An International Journal 1983;4(1):33‐7. - PubMed
Hepburn 1980 {published data only}
-
- Hepburn S. Method of local anesthesia for IUD insertion. Contraceptive Delivery Systems 1980;1(4):371‐82. - PubMed
Hollingworth 1995 {published data only}
-
- Hollingworth B. Pain control during insertion of intrauterine device. British Journal of Family Planning 1995;21(3):102‐3.
Jafari 2014 {published data only}
-
- Jafari A, Sayyahi M, Torkashvand R, Piryaei H. Effect of vitamin B1 on pain after insertion of intrauterine device [article in Persian]. Journal of Babol University of Medical Sciences 2014;16(3):13‐20.
Mirmohamad Aliei 2013 {published data only}
-
- Mirmohamad Aliei M, Khazaie F, Rahnama P, Rahimikian F, Modarres M, Bekhradi R, et al. Effect of lavender on pain during insertion of intrauterine device: A clinical trial [article in Persian]. Journal of Babol University of Medical Sciences 2013;15(4):93‐9.
-
- Mirmohammadali AM. Effect of lavender essential oil on anxiety and pain during insertion of intrauterine device. apps.who.int/trialsearch/trial.aspx?trialid=IRCT201209266206N4 (accessed 29 July 2014). [IRCT201209266206N4]
Newton 1977 {published data only}
-
- Newton JR, Reading AE. The effects of psychological preparation on pain at intrauterine device insertion. Contraception 1977;16(5):523‐32. - PubMed
Oloto 1997 {published data only}
-
- Oloto EJ, Bromham DR, Murty JA. Pain and discomfort perception at IUD insertion‐‐effect of short‐duration, low‐volume, intracervical application of two percent lignocaine gel (Instillagel™)‐‐a preliminary study. British Journal of Family Planning 1997;22(4):177‐80.
Stephenson 2010 {published data only}
-
- Stephenson J. Does topical anaesthetic gel reduce pain/discomfort during intrauterine device (IUD)/intrauterine system (IUD) insertion? A randomised, placebo‐controlled, double‐blind trial. www.controlled‐trials.com/ISRCTN83819888 (accessed 23 September 2014).
Teal 2012 {published data only}
-
- Teal S. IUD insertion in nulliparous women. clinicaltrials.gov/show/NCT01422226 (accessed 19 August 2014).
Thiery 1985 {published data only}
-
- Thiery M. Pain relief at insertion and removal of an IUD: a simplified technique for paracervical block. Advances in Contraception 1985;1(2):167‐70. - PubMed
References to studies awaiting assessment
Brody 2011 {published data only}
-
- Brody K. Topical Lidocaine on the Cervix and Intra‐Cervical Prior to Insertion of Intrauterine Devices (10‐053). http://clinicaltrials.gov/show/NCT01192490 (accessed 17 September 2014).
Elsafty 2011 {published data only}
-
- Elsafty ASE. Efficacy study of topical application of lidocaine spray prior to IUD insertion. clinicaltrials.gov/show/NCT01496105 (accessed 18 September 2014).
Khodokarami 2011 {published data only}
-
- Khodokarami N. The effect of chamomile essential oil on pain intensity of IUD insertion. www.irct.ir/searchresult.php?id=8950&number=1 (accessed 23 September 2014).
Singh 2015 {published data only}
-
- Singh R. Nitrous oxide for pain management of IUD insertion (NIUD). clinicaltrials.gov/ct2/show/NCT02391714 (accessed 4 May 2015). [DOI: ]
-
- Singh RH, Thaxton LD, Carr S, Leeman LM, Schneider E, Espey E. Nitrous oxide for intrauterine device insertion in nulliparous women: a randomized controlled trial [22]. Obstetrics and Gynecology 2015;125(Suppl 1):7S. - PubMed
References to ongoing studies
Fouda 2015 {published data only}
-
- Fouda UM. Diclofenac plus lidocaine gel for pain relief during intrauterine device insertion (IUD). clinicaltrials.gov/show/NCT02332057 (accessed 27 January 2015).
Jamshidi 2014 {published data only}
-
- Jamshidi R. Study of local anesthesia as a method to decrease IUD insertion related pain. clinicaltrials.gov/show/NCT01967017 (accessed 11 September 2013).
Mody 2014 {published data only}
-
- Mody S, Culwell K. Pain control for intrauterine device placement using paracervical block. clinicaltrials.gov/show/NCT02219308 (accessed 11 September 2014).
Additional references
Allen 2014
-
- Allen RH, Carey MS, Raker C, Goyal V, Matteson K. A prospective cohort study of pain with intrauterine device insertion among women with and without vaginal deliveries. Journal of Obstetrics and Gynaecology 2014;34(3):263‐7. - PubMed
Asker 2006
-
- Asker C, Stokes‐Lampard H, Beavan J, Wilson S. What is it about intrauterine devices that women find unacceptable? Factors that make women non‐users: a qualitative study. Journal of Family Planning and Reproductive Health Care 2006;32(2):89‐94. - PubMed
Bahamondes 2014
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Comm Adolescent Health 2012
-
- Committee on Adolescent Health Care, Long‐Acting Reversible Contraception Working Group. American College of Obstetricians Gynecologists Committee Opinion no. 539: Adolescents and long‐acting reversible contraception: implants and intrauterine devices. Obstetrics and Gynecology 2012;120(4):983‐8. - PubMed
Gemzell‐Danielsson 2013
Goldberg 2003
-
- Goldberg AB, Carusi DA, Meckstroth KR. Misoprostol in gynecology. Current Women's Health Reports 2003;3(6):475‐83. - PubMed
Goldstuck 1985
-
- Goldstuck ND, Mathews ML. A comparison of the actual and expected pain response following insertion of an intrauterine contraceptive device. Clinical Reproduction and Fertility 1985;3(1):65‐71. - PubMed
Grimes 2006
Grimes 2008
-
- Grimes DA, Mishell DR. Intrauterine contraception as an alternative to interval tubal sterilization. Contraception 2008;77(1):6‐9. - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org (accessed 25 March 2014).
Kaislasuo 2014
-
- Kaislasuo J, Heikinheimo O, Lähteenmäki P, Suhonen S. Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstetrics and Gynecology 2014;124(2 Pt 1):345‐53. - PubMed
Kass‐Wolff 2014
-
- Kass‐Wolff JH, Fisher JE. Evidence‐based pain management for endometrial biopsies and IUD insertions. Nurse Practitioner 2014;39(3):43‐50. - PubMed
Mercier 2012
Murty 2003
-
- Murty J. Use and effectiveness of oral analgesia when fitting an intrauterine device. Journal of Family Planning and Reproductive Health Care 2003;29(3):150‐1. - PubMed
Ott 2014
-
- Ott MA, Sucato GS, Committee on Adolescence. Contraception for adolescents. Pediatrics 2014;134(4):e1257‐81. - PubMed
Pergialiotis 2014
-
- Pergialiotis V, Vlachos DG, Protopappas A, Vlachos GD. Analgesic options for placement of an intrauterine contraceptive: a meta‐analysis. European Journal of Contraception and Reproductive Health Care 2014;19(3):149‐60. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Thomson 1997
-
- Thomson AJ, Lunan CB, Cameron AD, Cameron IT, Greer IA, Norman JE. Nitric oxide donors induce ripening of the human uterine cervix: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(9):1054‐1057. - PubMed
Tolcher 2003
-
- Tolcher R. Intrauterine techniques: contentious or consensus opinion?. Journal of Family Planning and Reproductive Health Care 2003;29(1):21‐4. - PubMed
Turok 2011
UN 2013
-
- United Nations Population Division. World Contraceptive Patterns 2013. www.un.org/en/development/desa/population/publications/family/contracept... (accessed 11 February 2015).
Waddington 2012
-
- Waddington A, Reid R. More harm than good: the lack of evidence for administering misoprostol prior to IUD insertion. Journal of Obstetrics and Gynaecology Canada 2012;34(12):1177‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

