Vascularisation of the central nervous system
- PMID: 26222953
- PMCID: PMC4678116
- DOI: 10.1016/j.mod.2015.07.001
Vascularisation of the central nervous system
Abstract
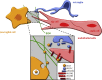
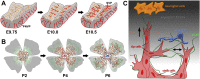
The developing central nervous system (CNS) is vascularised through the angiogenic invasion of blood vessels from a perineural vascular plexus, followed by continued sprouting and remodelling until a hierarchical vascular network is formed. Remarkably, vascularisation occurs without perturbing the intricate architecture of the neurogenic niches or the emerging neural networks. We discuss the mouse hindbrain, forebrain and retina as widely used models to study developmental angiogenesis in the mammalian CNS and provide an overview of key cellular and molecular mechanisms regulating the vascularisation of these organs.
Keywords: GPCR; Neuropilin; Semaphorin; VEGF; WNT.
Copyright © 2015. Published by Elsevier Ireland Ltd.
Figures

References
-
- Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonniere L., Bernard M., van Horssen J., de Vries H.E., Charron F., Prat A. The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. - PubMed
-
- Anderson K.D., Pan L., Yang X.-m., Hughes V.C., Walls J.R., Dominguez M.G., Simmons M.V., Burfeind P., Xue Y., Wei Y., Macdonald L.E., Thurston G., Daly C., Lin H.C., Economides A.N., Valenzuela D.M., Murphy A.J., Yancopoulos G.D., Gale N.W. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. 2011;108:2807–2812. - PMC - PubMed
-
- Armulik A., Genove G., Mae M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

