Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity
- PMID: 26223301
- PMCID: PMC4534720
- DOI: 10.1126/scitranslmed.aac5546
Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity
Abstract
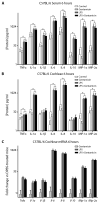
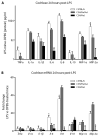
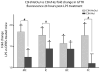
The ototoxic aminoglycoside antibiotics are essential to treat severe bacterial infections, particularly in neonatal intensive care units. Using a bacterial lipopolysaccharide (LPS) experimental model of sepsis, we tested whether LPS-mediated inflammation potentiates cochlear uptake of aminoglycosides and permanent hearing loss in mice. Using confocal microscopy and enzyme-linked immunosorbent assays, we found that low-dose LPS (endotoxemia) greatly increased cochlear concentrations of aminoglycosides and resulted in vasodilation of cochlear capillaries without inducing paracellular flux across the blood-labyrinth barrier (BLB) or elevating serum concentrations of the drug. Additionally, endotoxemia increased expression of both serum and cochlear inflammatory markers. These LPS-induced changes, classically mediated by Toll-like receptor 4 (TLR4), were attenuated in TLR4-hyporesponsive mice. Multiday dosing with aminoglycosides during chronic endotoxemia induced greater hearing threshold shifts and sensory cell loss compared to mice without endotoxemia. Thus, endotoxemia-mediated inflammation enhanced aminoglycoside trafficking across the BLB and potentiated aminoglycoside-induced ototoxicity. These data indicate that patients with severe infections are at greater risk of aminoglycoside-induced hearing loss than previously recognized.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

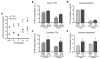


Comment in
-
Drug-induced hearing loss: Infection raises the odds.Sci Transl Med. 2015 Jul 29;7(298):298fs31. doi: 10.1126/scitranslmed.aac9811. Sci Transl Med. 2015. PMID: 26223298
-
Finding ways to solve or prevent aminoglycoside-induced ototoxicity?Ann Transl Med. 2016 Dec;4(24):533. doi: 10.21037/atm.2016.11.71. Ann Transl Med. 2016. PMID: 28149894 Free PMC article. No abstract available.
References
-
- Kimberlin DW. Meningitis in the Neonate. Current treatment options in neurology. 2002;4:239–248. - PubMed
-
- Radigan EA, Gilchrist NA, Miller MA. Management of aminoglycosides in the intensive care unit. Journal of intensive care medicine. 2010;25:327–342. - PubMed
-
- Cheng AG, Johnston PR, Luz J, Uluer A, Fligor B, Licameli GR, Kenna MA, Jones DT. Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngol Head Neck Surg. 2009;141:86–90. - PubMed
-
- Pillers DM, Schleiss MR. Gentamicin in the Clinical Setting. The Volta Review. 2005;105:205–210.
-
- Erenberg A, Lemons J, Sia C, Trunkel D, Ziring P. Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics. Task Force on Newborn and Infant Hearing, 1998–1999. Pediatrics. 1999;103:527–530. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

