The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held
- PMID: 26223835
- PMCID: PMC4594243
- DOI: 10.1113/JP270849
The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held
Abstract
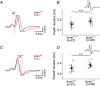
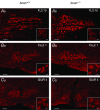
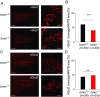
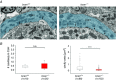
Key points: The proteoglycan brevican is a major component of the extracellular matrix of perineuronal nets and is highly enriched in the perisynaptic space suggesting a role for synaptic transmission. We have introduced the calyx of Held in the auditory brainstem as a model system to study the impact of brevican on dynamics and reliability of synaptic transmission. In vivo extracellular single-unit recordings at the calyx of Held in brevican-deficient mice yielded a significant increase in the action potential (AP) transmission delay and a prolongation of pre- and postsynaptic APs. The changes in dynamics of signal transmission were accompanied by the reduction of presynaptic vGlut1 and ultrastructural changes in the perisynaptic space. These data show that brevican is an important mediator of fast synaptic transmission at the calyx of Held.
Abstract: The extracellular matrix is an integral part of the neural tissue. Its most conspicuous manifestation in the brain are the perineuronal nets (PNs) which surround somata and proximal dendrites of distinct neuron types. The chondroitin sulfate proteoglycan brevican is a major component of PNs. In contrast to other PN-comprising proteoglycans (e.g. aggrecan and neurocan), brevican is mainly expressed in the perisynaptic space closely associated with both the pre- and postsynaptic membrane. This specific localization prompted the hypothesis that brevican might play a role in synaptic transmission. In the present study we specifically investigated the role of brevican in synaptic transmission at a central synapse, the calyx of Held in the medial nucleus of the trapezoid body, by the use of in vivo electrophysiology, immunohistochemistry, biochemistry and electron microscopy. In vivo extracellular single-unit recordings were acquired in brevican-deficient mice and the dynamics and reliability of synaptic transmission were compared to wild-type littermates. In knockout mice, the speed of pre-to-postsynaptic action potential (AP) transmission was reduced and the duration of the respective pre- and postsynaptic APs increased. The reliability of signal transmission, however, was not affected by the lack of brevican. The changes in dynamics of signal transmission were accompanied by the reduction of (i) presynaptic vGlut1 and (ii) the size of subsynaptic cavities. The present results suggest an essential role of brevican for the functionality of high-speed synaptic transmission at the calyx of Held.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures





References
-
- Bergles DE & Edwards RH (2008). The role of glutamate transporters in synaptic transmission In Structural and Functional Organization of the Synapse, ed. Hell JW, Ehlers & Michael D, pp. 23–61. Springer, New York.
-
- Billups B (2005). Colocalization of vesicular glutamate transporters in the rat superior olivary complex. Neurosci Lett 382, 66–70. - PubMed
-
- Blaesse P, Ehrhardt S, Friauf E & Nothwang HG (2005). Developmental pattern of three vesicular glutamate transporters in the rat superior olivary complex. Cell Tissue Res 320, 33–50. - PubMed
-
- Blosa M, Sonntag M, Brückner G, Jäger C, Seeger G, Matthews RT, Rübsamen R, Arendt T & Morawski M (2013). Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body–implications for physiological functions. Neuroscience 228, 215–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

