Rapid Column-Free Enrichment of Mononuclear Cells from Solid Tissues
- PMID: 26223896
- PMCID: PMC4519776
- DOI: 10.1038/srep12490
Rapid Column-Free Enrichment of Mononuclear Cells from Solid Tissues
Abstract
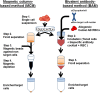
We have developed a rapid negative selection method to enrich rare mononuclear cells from human tissues. Unwanted and antibody-tethered cells are selectively depleted during a Ficoll separation step, and there is no need for magnetic-based reagents and equipment. The new method is fast, customizable, inexpensive, remarkably efficient, and easy to perform, and per sample the overall cost is less than one-tenth the cost associated with a magnetic column-based method.
Figures

Similar articles
-
Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient.Curr Protoc Stem Cell Biol. 2007 Jun;Chapter 2:Unit 2A.1. doi: 10.1002/9780470151808.sc02a01s1. Curr Protoc Stem Cell Biol. 2007. PMID: 18785173
-
Separation of leukocytes from blood using spiral channel with trapezoid cross-section.Anal Chem. 2012 Nov 6;84(21):9324-31. doi: 10.1021/ac302085y. Epub 2012 Oct 12. Anal Chem. 2012. PMID: 23025404
-
Monocyte enrichment from leukapheresis products by using the Elutra cell separator.Transfusion. 2007 Dec;47(12):2290-6. doi: 10.1111/j.1537-2995.2007.01470.x. Epub 2007 Aug 30. Transfusion. 2007. PMID: 17764512
-
Processing of bone marrow for transplantation: development of a mononuclear cell enrichment protocol.Prog Clin Biol Res. 1994;389:695-704. Prog Clin Biol Res. 1994. PMID: 7700937 No abstract available.
-
Evaluation of a robotic system for the recovery of peripheral blood mononuclear cells.Biologicals. 2012 Jan;40(1):31-5. doi: 10.1016/j.biologicals.2011.09.009. Epub 2011 Oct 19. Biologicals. 2012. PMID: 22014410
Cited by
-
A Progenitor Cell Expressing Transcription Factor RORγt Generates All Human Innate Lymphoid Cell Subsets.Immunity. 2016 May 17;44(5):1140-50. doi: 10.1016/j.immuni.2016.04.007. Epub 2016 May 10. Immunity. 2016. PMID: 27178467 Free PMC article.
-
Aiolos represses CD4+ T cell cytotoxic programming via reciprocal regulation of TFH transcription factors and IL-2 sensitivity.Nat Commun. 2023 Mar 24;14(1):1652. doi: 10.1038/s41467-023-37420-0. Nat Commun. 2023. PMID: 36964178 Free PMC article.
-
CD56 Expression Marks Human Group 2 Innate Lymphoid Cell Divergence from a Shared NK Cell and Group 3 Innate Lymphoid Cell Developmental Pathway.Immunity. 2018 Sep 18;49(3):464-476.e4. doi: 10.1016/j.immuni.2018.08.010. Epub 2018 Sep 4. Immunity. 2018. PMID: 30193847 Free PMC article.
-
NKp80 Defines a Critical Step during Human Natural Killer Cell Development.Cell Rep. 2016 Jul 12;16(2):379-391. doi: 10.1016/j.celrep.2016.05.095. Epub 2016 Jun 30. Cell Rep. 2016. PMID: 27373165 Free PMC article.
-
Single-cell RNA sequencing identifies a population of human liver-type ILC1s.Cell Rep. 2023 Jan 31;42(1):111937. doi: 10.1016/j.celrep.2022.111937. Epub 2023 Jan 1. Cell Rep. 2023. PMID: 36640314 Free PMC article.
References
-
- Mjösberg J. M. et al.. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12, 1055–1062 (2011). - PubMed
-
- Ferlazzo G. & Münz C. NK cell compartments and their activation by dendritic cells. J Immunol 172, 1333–1339 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

