HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma
- PMID: 26224068
- PMCID: PMC4518626
- DOI: 10.1186/s12974-015-0360-2
HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma
Abstract
Background: Acute glaucoma is a significantly sight-threatening cause of irreversible blindness in the world characterized by a sudden and substantial intraocular pressure (IOP) increase and subsequent retinal ganglion cell (RGC) death. This study aims to explore the role of high-mobility group box 1 (HMGB1) in an acute glaucoma mouse model.
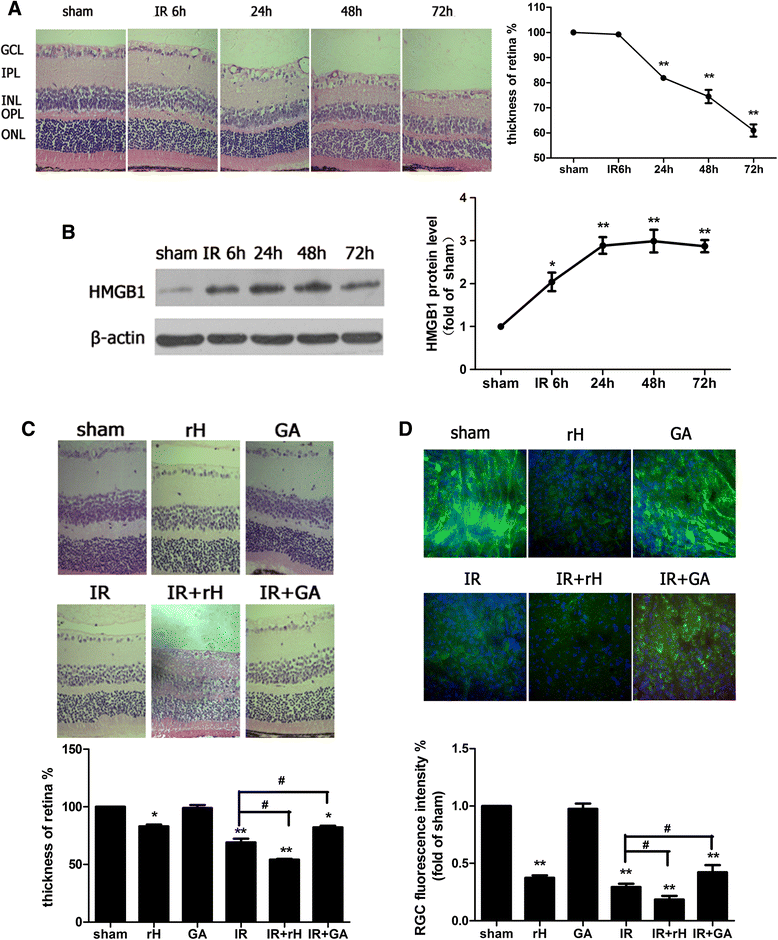
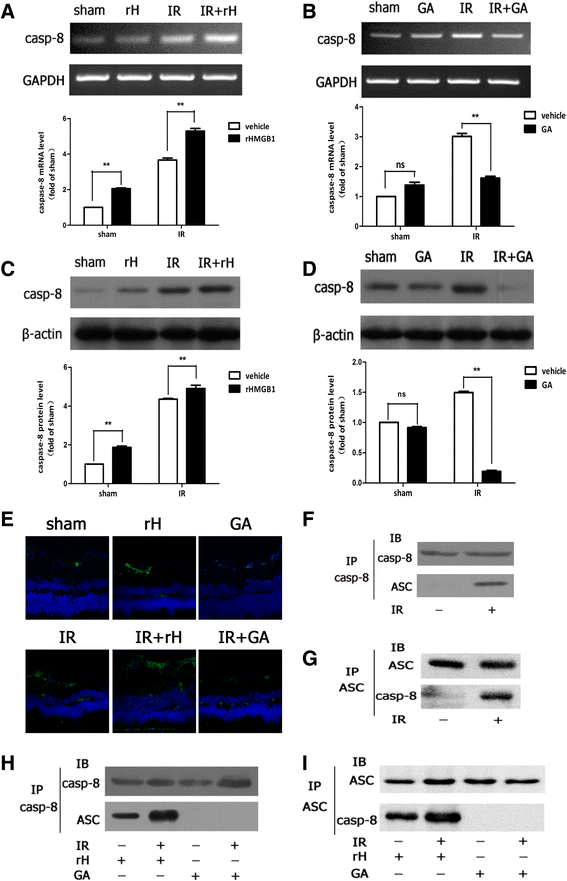
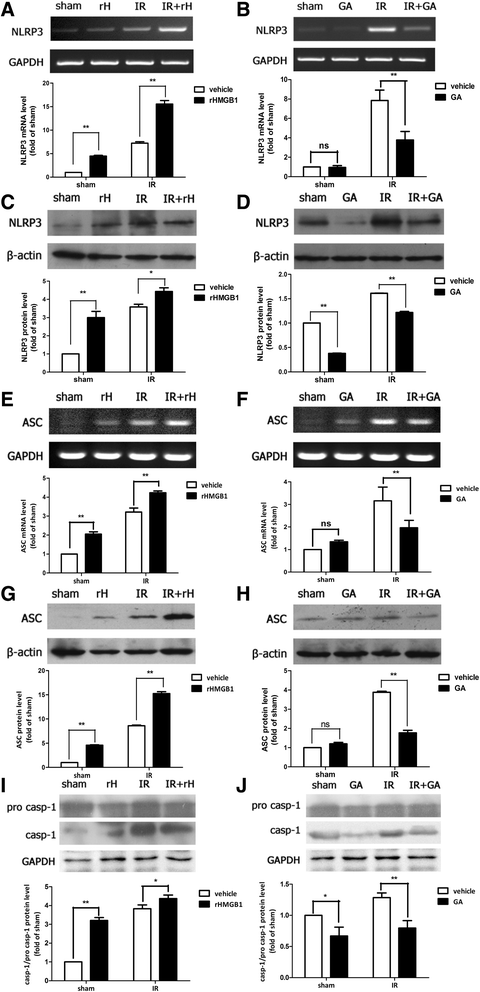
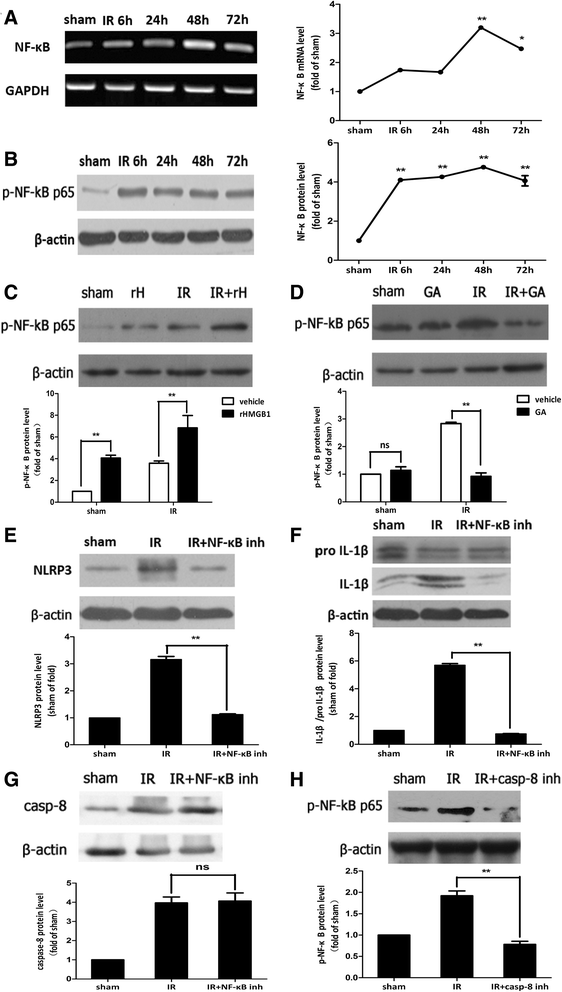
Methods: An acute glaucoma model was induced by a rapid and substantial increase IOP to 70 mmHg for 60 min via anterior chamber punctured and affused with Balance Salt Solution in C57BL/6 mice. Retinal tissue ischemic damage and loss of RGCs were assessed at 6, 24, 48, 72 h after high IOP treatment, and at 48 h, group with or without recombinant high-mobility group box 1 (rHMGB1), the HMGB1 inhibitor, glycyrrhizic acid (GA), and by HE and immunofluorescent staining. The nuclear factor κB (NF-κB) inhibitor, JSH-23, and caspase-8 inhibitor, Z-IETD-fmk, were injected into vitreous. Reverse transcription and semi-quantitative reverse transcription polymerase chain reaction (RT-PCR), western blotting, and immunoprecipitation were performed to evaluate the expression level of nucleotide-binding domain, leucine-rich repeat containing protein 3 (NLRP3), phosphor-NF-κB p65, caspase-8, caspase-1, apoptosis-associated speck-like protein containing a CARD (ASC), and interleukin-1β (IL-1β).
Results: HMGB1 was increased in ischemic retinal tissue during acute glaucoma as early as 6 h after rapid IOP elevation. Exogenous HMGB1 exacerbated retinal ischemic damage, RGC loss, and inhibition of endogenous HMGB1 significantly reduced the severity of disease. HMGB1 significantly induced the elevation of canonical NLRP3, ASC, caspase-1, and non-canonical capase-8-ASC inflammasome and promoted the processing of IL-1β. Furthermore, the effect of HMGB1 on NLRP3 inflammasome activation and IL-1β production was dependent on NF-κB pathway. Thus, HMGB1/caspase-8 pathway promoted the processing of IL-1β via NF-κB pathway.
Conclusion: The findings of this study identified a novel signaling pathway in which HMGB1, in response to acutely elevated intraocular pressure, activated the canonical NLRP3 and non-canonical caspase-8 inflammasomes and production of IL-1β during acute glaucoma development. These results provide new insights to the understanding of the innate response that contributes to pathogenesis of acute glaucoma.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

