Galectin-Binding O-Glycosylations as Regulators of Malignancy
- PMID: 26224120
- PMCID: PMC4537818
- DOI: 10.1158/0008-5472.CAN-15-0834
Galectin-Binding O-Glycosylations as Regulators of Malignancy
Abstract
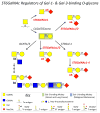
Cancer cells commonly display aberrant surface glycans and related glycoconjugate scaffolds. Compared with their normal counterparts, cancer cell glycans are variably produced and often structurally distinct, serving as biomarkers of cancer progression or as functional entities to malignancy. The glycan signature of a cancer cell is created by the collaborative activities of glycosyltransferases, glycosidases, nucleotide-sugar transporters, sulfotransferases, and glycan-bearing protein/lipid scaffolds. In a coordinated fashion, these factors regulate the synthesis of cancer cell glycans and thus are considered correlates of cancer cell behavior. Functionally, cancer cell glycans can serve as binding targets for endogenous lectin effectors, such as C-type selectins and S-type galectins. There has been a recent surge of important observations of the role of glycosytransferases, specifically α2,6 sialyltransferases, in regulating the length and lectin-binding features of serine/threonine (O)-glycans found on cancer cells. The capping activity of O-glycan-specific α2,6 sialyltransferases, in particular, has been found to regulate cancer growth and metastasis in a galectin-dependent manner. These findings highlight the functional importance of cancer cell O-glycans and related galectin-binding features in the virulent activity of cancer and raise the prospect of targeting cancer cell glycans as effective anticancer therapeutics.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322–35. - PubMed
-
- Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert reviews in molecular medicine. 2008;10:e17. - PubMed
-
- Tinari N, Kuwabara I, Huflejt ME, Shen PF, Iacobelli S, Liu FT. Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. International journal of cancer Journal international du cancer. 2001;91:167–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

