Inflammation-induced desmoglein-2 ectodomain shedding compromises the mucosal barrier
- PMID: 26224314
- PMCID: PMC4569309
- DOI: 10.1091/mbc.E15-03-0147
Inflammation-induced desmoglein-2 ectodomain shedding compromises the mucosal barrier
Abstract
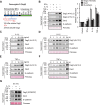
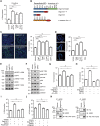
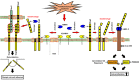
Desmosomal cadherins mediate intercellular adhesion and control epithelial homeostasis. Recent studies show that proteinases play an important role in the pathobiology of cancer by targeting epithelial intercellular junction proteins such as cadherins. Here we describe the proinflammatory cytokine-induced activation of matrix metalloproteinase 9 and a disintegrin and metalloproteinase domain-containing protein 10, which promote the shedding of desmosomal cadherin desmoglein-2 (Dsg2) ectodomains in intestinal epithelial cells. Epithelial exposure to Dsg2 ectodomains compromises intercellular adhesion by promoting the relocalization of endogenous Dsg2 and E-cadherin from the plasma membrane while also promoting proliferation by activation of human epidermal growth factor receptor 2/3 signaling. Cadherin ectodomains were detected in the inflamed intestinal mucosa of mice with colitis and patients with ulcerative colitis. Taken together, our findings reveal a novel response pathway in which inflammation-induced modification of columnar epithelial cell cadherins decreases intercellular adhesion while enhancing cellular proliferation, which may serve as a compensatory mechanism to promote repair.
© 2015 Kamekura et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



Similar articles
-
Loss of Desmoglein 2 Contributes to the Pathogenesis of Crohn's Disease.Inflamm Bowel Dis. 2015 Oct;21(10):2349-59. doi: 10.1097/MIB.0000000000000486. Inflamm Bowel Dis. 2015. PMID: 26115074
-
EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2.Mol Biol Cell. 2009 Jan;20(1):328-37. doi: 10.1091/mbc.e08-04-0356. Epub 2008 Nov 5. Mol Biol Cell. 2009. PMID: 18987342 Free PMC article.
-
Desmoglein 2, but not desmocollin 2, protects intestinal epithelia from injury.Mucosal Immunol. 2018 Nov;11(6):1630-1639. doi: 10.1038/s41385-018-0062-z. Epub 2018 Aug 16. Mucosal Immunol. 2018. PMID: 30115995
-
Targeting desmosomal adhesion and signalling for intestinal barrier stabilization in inflammatory bowel diseases-Lessons from experimental models and patients.Acta Physiol (Oxf). 2021 Jan;231(1):e13492. doi: 10.1111/apha.13492. Epub 2020 May 22. Acta Physiol (Oxf). 2021. PMID: 32419327 Review.
-
Cleavage of transmembrane junction proteins and their role in regulating epithelial homeostasis.Tissue Barriers. 2013 Apr 1;1(2):e24783. doi: 10.4161/tisb.24783. Tissue Barriers. 2013. PMID: 24665393 Free PMC article. Review.
Cited by
-
Desmoglein-2 Affects Vascular Function in Moyamoya Disease by Interacting with MMP-9 and Influencing PI3K Signaling.Mol Neurobiol. 2024 Sep;61(9):6539-6552. doi: 10.1007/s12035-024-04010-0. Epub 2024 Feb 7. Mol Neurobiol. 2024. PMID: 38326520 Free PMC article.
-
Desmoglein-2 as a cancer modulator: friend or foe?Front Oncol. 2023 Dec 22;13:1327478. doi: 10.3389/fonc.2023.1327478. eCollection 2023. Front Oncol. 2023. PMID: 38188287 Free PMC article. Review.
-
Serum DSG2 as a potential biomarker for diagnosis of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma.Biosci Rep. 2022 May 27;42(5):BSR20212612. doi: 10.1042/BSR20212612. Biosci Rep. 2022. PMID: 35521959 Free PMC article.
-
Desmocollin-2 promotes intestinal mucosal repair by controlling integrin-dependent cell adhesion and migration.Mol Biol Cell. 2020 Mar 15;31(6):407-418. doi: 10.1091/mbc.E19-12-0692. Epub 2020 Jan 22. Mol Biol Cell. 2020. PMID: 31967937 Free PMC article.
-
Identification of Desmoglein-2 as a novel target of Helicobacter pylori HtrA in epithelial cells.Cell Commun Signal. 2021 Nov 6;19(1):108. doi: 10.1186/s12964-021-00788-x. Cell Commun Signal. 2021. PMID: 34742300 Free PMC article.
References
-
- Amagai M, Yamaguchi T, Hanakawa Y, Nishifuji K, Sugai M, Stanley JR. Staphylococcal exfoliative toxin B specifically cleaves desmoglein 1. J Invest Dermatol. 2002;118:845–850. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. - PubMed
-
- Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

