Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer
- PMID: 26224368
- PMCID: PMC4644680
- DOI: 10.1158/1541-7786.MCR-15-0204
Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer
Abstract
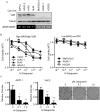
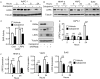
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy in need of more effective treatment approaches. One potential therapeutic target is Wnt/β-catenin signaling, which plays important roles in PDAC tumor initiation and progression. Among Wnt inhibitors with suitable in vivo biologic activity is vitamin D, which is known to antagonize Wnt/β-catenin signaling in colorectal cancer and have antitumor activity in PDAC. For this study, the relationship between vitamin D signaling, Wnt/β-catenin activity, and tumor cell growth in PDAC was investigated through the use of calcipotriol, a potent non-hypercalcemic vitamin D analogue. PDAC tumor cell growth inhibition by calcipotriol was positively correlated with vitamin D receptor expression and Wnt/β-catenin activity. Furthermore, vitamin D and Wnt signaling activity were found to be reciprocally linked through feedback regulation. Calcipotriol inhibited autocrine Wnt/β-catenin signaling in PDAC cell lines in parallel with decreased protein levels of the low-density lipoprotein receptor-related protein 6 (LRP6), a requisite coreceptor for ligand-dependent canonical Wnt signaling. Decrease in LRP6 protein seen with calcipotriol was mediated through a novel mechanism involving transcriptional upregulation of low-density lipoprotein receptor adaptor protein 1 (LDLRAP1). Finally, changes in LRP6 or LDLRAP1 expression directly altered Wnt reporter activity, supporting their roles as regulators of ligand-dependent Wnt/β-catenin signaling.
Implications: This study provides a novel biochemical target through which vitamin D signaling exerts inhibitory effects on Wnt/β-catenin signaling, as well as potential biomarkers for predicting and following tumor response to vitamin D-based therapy.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway.PLoS One. 2011;6(12):e29290. doi: 10.1371/journal.pone.0029290. Epub 2011 Dec 16. PLoS One. 2011. PMID: 22195040 Free PMC article.
-
WNT7B mediates autocrine Wnt/β-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma.Oncogene. 2014 Feb 13;33(7):899-908. doi: 10.1038/onc.2013.23. Epub 2013 Feb 18. Oncogene. 2014. PMID: 23416978 Free PMC article.
-
Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin and mTORC1 signaling in prostate and breast cancer cells.Cell Signal. 2014 Jun;26(6):1303-9. doi: 10.1016/j.cellsig.2014.02.018. Epub 2014 Mar 6. Cell Signal. 2014. PMID: 24607787 Free PMC article.
-
β-Catenin-Independent Roles of Wnt/LRP6 Signaling.Trends Cell Biol. 2016 Dec;26(12):956-967. doi: 10.1016/j.tcb.2016.07.009. Epub 2016 Aug 24. Trends Cell Biol. 2016. PMID: 27568239 Review.
-
Targeting LRP6: A new strategy for cancer therapy.Pharmacol Res. 2024 Jun;204:107200. doi: 10.1016/j.phrs.2024.107200. Epub 2024 May 6. Pharmacol Res. 2024. PMID: 38710241 Review.
Cited by
-
Tumor-reducing effect of the clinically used drug clofazimine in a SCID mouse model of pancreatic ductal adenocarcinoma.Oncotarget. 2017 Jun 13;8(24):38276-38293. doi: 10.18632/oncotarget.11299. Oncotarget. 2017. PMID: 27542263 Free PMC article.
-
Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms.Nutrients. 2022 Mar 30;14(7):1448. doi: 10.3390/nu14071448. Nutrients. 2022. PMID: 35406059 Free PMC article. Review.
-
A Role for the WNT Co-Receptor LRP6 in Pathogenesis and Therapy of Epithelial Cancers.Cancers (Basel). 2019 Aug 13;11(8):1162. doi: 10.3390/cancers11081162. Cancers (Basel). 2019. PMID: 31412666 Free PMC article. Review.
-
miR-202-3p Regulates Sertoli Cell Proliferation, Synthesis Function, and Apoptosis by Targeting LRP6 and Cyclin D1 of Wnt/β-Catenin Signaling.Mol Ther Nucleic Acids. 2019 Mar 1;14:1-19. doi: 10.1016/j.omtn.2018.10.012. Epub 2018 Oct 25. Mol Ther Nucleic Acids. 2019. PMID: 30513418 Free PMC article.
-
DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts.Cells. 2020 Jan 17;9(1):236. doi: 10.3390/cells9010236. Cells. 2020. PMID: 31963554 Free PMC article.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R01 HL105278/HL/NHLBI NIH HHS/United States
- HL088093/HL/NHLBI NIH HHS/United States
- P01 DK098108/DK/NIDDK NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- T32 CA009370/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- R37 DK057978/DK/NIDDK NIH HHS/United States
- HL105278/HL/NHLBI NIH HHS/United States
- DK057978/DK/NIDDK NIH HHS/United States
- R24 DK090962/DK/NIDDK NIH HHS/United States
- DK090962/DK/NIDDK NIH HHS/United States
- P01 CA163200/CA/NCI NIH HHS/United States
- ES010337/ES/NIEHS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 CA009056/CA/NCI NIH HHS/United States
- R01 DK057978/DK/NIDDK NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States

