Cell shape regulation through mechanosensory feedback control
- PMID: 26224568
- PMCID: PMC4535415
- DOI: 10.1098/rsif.2015.0512
Cell shape regulation through mechanosensory feedback control
Abstract
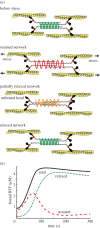
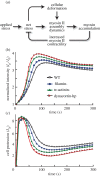
Cells undergo controlled changes in morphology in response to intracellular and extracellular signals. These changes require a means for sensing and interpreting the signalling cues, for generating the forces that act on the cell's physical material, and a control system to regulate this process. Experiments on Dictyostelium amoebae have shown that force-generating proteins can localize in response to external mechanical perturbations. This mechanosensing, and the ensuing mechanical feedback, plays an important role in minimizing the effect of mechanical disturbances in the course of changes in cell shape, especially during cell division, and likely in other contexts, such as during three-dimensional migration. Owing to the complexity of the feedback system, which couples mechanical and biochemical signals involved in shape regulation, theoretical approaches can guide further investigation by providing insights that are difficult to decipher experimentally. Here, we present a computational model that explains the different mechanosensory and mechanoresponsive behaviours observed in Dictyostelium cells. The model features a multiscale description of myosin II bipolar thick filament assembly that includes cooperative and force-dependent myosin-actin binding, and identifies the feedback mechanisms hidden in the observed mechanoresponsive behaviours of Dictyostelium cells during micropipette aspiration experiments. These feedbacks provide a mechanistic explanation of cellular retraction and hence cell shape regulation.
Keywords: cell shape regulation; force feedback; mechanosensing; myosin II.
© 2015 The Author(s).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
