CYLD and the NEMO Zinc Finger Regulate Tumor Necrosis Factor Signaling and Early Embryogenesis
- PMID: 26224629
- PMCID: PMC4571959
- DOI: 10.1074/jbc.M115.658096
CYLD and the NEMO Zinc Finger Regulate Tumor Necrosis Factor Signaling and Early Embryogenesis
Abstract
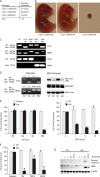
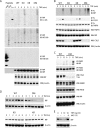
NF-κB essential modulator (NEMO) and cylindromatosis protein (CYLD) are intracellular proteins that regulate the NF-κB signaling pathway. Although mice with either CYLD deficiency or an alteration in the zinc finger domain of NEMO (K392R) are born healthy, we found that the combination of these two gene defects in double mutant (DM) mice is early embryonic lethal but can be rescued by the absence of TNF receptor 1 (TNFR1). Notably, NEMO was not recruited into the TNFR1 complex of DM cells, and consequently NF-κB induction by TNF was severely impaired and DM cells were sensitized to TNF-induced cell death. Interestingly, the TNF signaling defects can be fully rescued by reconstitution of DM cells with CYLD lacking ubiquitin hydrolase activity but not with CYLD mutated in TNF receptor-associated factor 2 (TRAF2) or NEMO binding sites. Therefore, our data demonstrate an unexpected non-catalytic function for CYLD as an adapter protein between TRAF2 and the NEMO zinc finger that is important for TNF-induced NF-κB signaling during embryogenesis.
Keywords: CYLD; NEMO; NF-κB (NF-KB); cell signaling; immunodeficiency; protein complex; tumor necrosis factor (TNF).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
MST1 Negatively Regulates TNFα-Induced NF-κB Signaling through Modulating LUBAC Activity.Mol Cell. 2019 Mar 21;73(6):1138-1149.e6. doi: 10.1016/j.molcel.2019.01.022. Epub 2019 Feb 21. Mol Cell. 2019. PMID: 30901564
-
Brefeldin A-Inhibited Guanine Nucleotide-Exchange Factor 1 (BIG1) Governs the Recruitment of Tumor Necrosis Factor Receptor-Associated Factor 2 (TRAF2) to Tumor Necrosis Factor Receptor 1 (TNFR1) Signaling Complexes.Int J Mol Sci. 2016 Nov 9;17(11):1869. doi: 10.3390/ijms17111869. Int J Mol Sci. 2016. PMID: 27834853 Free PMC article.
-
IRF-1 inhibits NF-κB activity, suppresses TRAF2 and cIAP1 and induces breast cancer cell specific growth inhibition.Cancer Biol Ther. 2015;16(7):1029-41. doi: 10.1080/15384047.2015.1046646. Cancer Biol Ther. 2015. PMID: 26011589 Free PMC article.
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
-
CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes.Cell Death Differ. 2010 Jan;17(1):25-34. doi: 10.1038/cdd.2009.43. Cell Death Differ. 2010. PMID: 19373246 Free PMC article. Review.
Cited by
-
All-atom molecular dynamics comparison of disease-associated zinc fingers.J Biomol Struct Dyn. 2018 Aug;36(10):2581-2594. doi: 10.1080/07391102.2017.1363662. Epub 2017 Oct 3. J Biomol Struct Dyn. 2018. PMID: 28814200 Free PMC article.
-
Prion protein is required for tumor necrosis factor α (TNFα)-triggered nuclear factor κB (NF-κB) signaling and cytokine production.J Biol Chem. 2017 Nov 17;292(46):18747-18759. doi: 10.1074/jbc.M117.787283. Epub 2017 Sep 12. J Biol Chem. 2017. PMID: 28900035 Free PMC article.
-
The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis.Nat Med. 2018 Feb;24(2):213-223. doi: 10.1038/nm.4461. Epub 2018 Jan 1. Nat Med. 2018. PMID: 29291351
-
In the moonlight: non-catalytic functions of ubiquitin and ubiquitin-like proteases.Front Mol Biosci. 2024 Feb 22;11:1349509. doi: 10.3389/fmolb.2024.1349509. eCollection 2024. Front Mol Biosci. 2024. PMID: 38455765 Free PMC article. Review.
-
The role of K63-linked polyubiquitination in cardiac hypertrophy.J Cell Mol Med. 2018 Oct;22(10):4558-4567. doi: 10.1111/jcmm.13669. Epub 2018 Aug 13. J Cell Mol Med. 2018. PMID: 30102008 Free PMC article. Review.
References
-
- Ware C. F. (2003) The TNF superfamily. Cytokine Growth Factor Rev. 14, 181–184 - PubMed
-
- Cao Z., Tanaka M., Regnier C., Rothe M., Yamit-hezi A., Woronicz J. D., Fuentes M. E., Durnin M. H., Dalrymple S. A., Goeddel D. V. (1999) NF-κB activation by tumor necrosis factor and interleukin-1. Cold Spring Harb. Symp. Quant. Biol. 64, 473–483 - PubMed
-
- Wertz I. E., Dixit V. M. (2008) Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 19, 313–324 - PubMed
-
- Brummelkamp T. R., Nijman S. M., Dirac A. M., Bernards R. (2003) Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

