Evidence that the DNA mismatch repair system removes 1-nucleotide Okazaki fragment flaps
- PMID: 26224637
- PMCID: PMC4591796
- DOI: 10.1074/jbc.M115.660357
Evidence that the DNA mismatch repair system removes 1-nucleotide Okazaki fragment flaps
Abstract
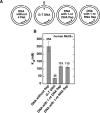
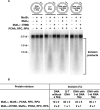
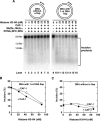
The DNA mismatch repair (MMR) system plays a major role in promoting genome stability and suppressing carcinogenesis. In this work, we investigated whether the MMR system is involved in Okazaki fragment maturation. We found that in the yeast Saccharomyces cerevisiae, the MMR system and the flap endonuclease Rad27 act in overlapping pathways that protect the nuclear genome from 1-bp insertions. In addition, we determined that purified yeast and human MutSα proteins recognize 1-nucleotide DNA and RNA flaps. In reconstituted human systems, MutSα, proliferating cell nuclear antigen, and replication factor C activate MutLα endonuclease to remove the flaps. ATPase and endonuclease mutants of MutLα are defective in the flap removal. These results suggest that the MMR system contributes to the removal of 1-nucleotide Okazaki fragment flaps.
Keywords: DNA endonuclease; DNA mismatch repair; DNA replication; Okazaki fragment maturation; cancer; genomic instability; mutL homolog 1 (MLH1).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Rad27 and Exo1 function in different excision pathways for mismatch repair in Saccharomyces cerevisiae.Nat Commun. 2021 Sep 22;12(1):5568. doi: 10.1038/s41467-021-25866-z. Nat Commun. 2021. PMID: 34552065 Free PMC article.
-
The mismatch repair and meiotic recombination endonuclease Mlh1-Mlh3 is activated by polymer formation and can cleave DNA substrates in trans.PLoS Biol. 2017 Apr 28;15(4):e2001164. doi: 10.1371/journal.pbio.2001164. eCollection 2017 Apr. PLoS Biol. 2017. PMID: 28453523 Free PMC article.
-
Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease.J Biol Chem. 2007 Dec 21;282(51):37181-90. doi: 10.1074/jbc.M707617200. Epub 2007 Oct 19. J Biol Chem. 2007. PMID: 17951253 Free PMC article.
-
Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.Eur J Cancer Prev. 2014 Jul;23(4):246-57. doi: 10.1097/CEJ.0000000000000019. Eur J Cancer Prev. 2014. PMID: 24614649 Review.
-
DNA mismatch repair and mutation avoidance pathways.J Cell Physiol. 2002 Apr;191(1):28-41. doi: 10.1002/jcp.10077. J Cell Physiol. 2002. PMID: 11920679 Review.
Cited by
-
DNA Mismatch Repair Interacts with CAF-1- and ASF1A-H3-H4-dependent Histone (H3-H4)2 Tetramer Deposition.J Biol Chem. 2016 Apr 22;291(17):9203-17. doi: 10.1074/jbc.M115.713271. Epub 2016 Mar 4. J Biol Chem. 2016. PMID: 26945061 Free PMC article.
-
Rad27 and Exo1 function in different excision pathways for mismatch repair in Saccharomyces cerevisiae.Nat Commun. 2021 Sep 22;12(1):5568. doi: 10.1038/s41467-021-25866-z. Nat Commun. 2021. PMID: 34552065 Free PMC article.
-
High-fidelity DNA ligation enforces accurate Okazaki fragment maturation during DNA replication.Nat Commun. 2021 Jan 20;12(1):482. doi: 10.1038/s41467-020-20800-1. Nat Commun. 2021. PMID: 33473124 Free PMC article.
-
The Major Replicative Histone Chaperone CAF-1 Suppresses the Activity of the DNA Mismatch Repair System in the Cytotoxic Response to a DNA-methylating Agent.J Biol Chem. 2016 Dec 30;291(53):27298-27312. doi: 10.1074/jbc.M116.760561. Epub 2016 Nov 21. J Biol Chem. 2016. PMID: 27872185 Free PMC article.
-
MutSα maintains the mismatch repair capability by inhibiting PCNA unloading.Elife. 2016 Jul 12;5:e15155. doi: 10.7554/eLife.15155. Elife. 2016. PMID: 27402201 Free PMC article.
References
-
- Modrich P. (1995) Mismatch repair, genetic stability and tumour avoidance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 347, 89–95 - PubMed
-
- Harfe B. D., Jinks-Robertson S. (2000) DNA mismatch repair and genetic instability. Annu. Rev. Genet. 34, 359–399 - PubMed
-
- Kunkel T. A., Erie D. A. (2005) DNA mismatch repair. Annu. Rev. Biochem. 74, 681–710 - PubMed
-
- Li G. M. (2008) Mechanisms and functions of DNA mismatch repair. Cell Res. 18, 85–98 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

