Immunodeficiency and severe susceptibility to bacterial infection associated with a loss-of-function homozygous mutation of MKL1
- PMID: 26224645
- PMCID: PMC4671330
- DOI: 10.1182/blood-2014-12-611012
Immunodeficiency and severe susceptibility to bacterial infection associated with a loss-of-function homozygous mutation of MKL1
Abstract
Megakaryoblastic leukemia 1 (MKL1), also known as MAL or myocardin-related transcription factor A (MRTF-A), is a coactivator of serum response factor, which regulates transcription of actin and actin cytoskeleton-related genes. MKL1 is known to be important for megakaryocyte differentiation and function in mice, but its role in immune cells is unexplored. Here we report a patient with a homozygous nonsense mutation in the MKL1 gene resulting in immunodeficiency characterized predominantly by susceptibility to severe bacterial infection. We show that loss of MKL1 protein expression causes a dramatic loss of filamentous actin (F-actin) content in lymphoid and myeloid lineage immune cells and widespread cytoskeletal dysfunction. MKL1-deficient neutrophils displayed reduced phagocytosis and almost complete abrogation of migration in vitro. Similarly, primary dendritic cells were unable to spread normally or to form podosomes. Silencing of MKL1 in myeloid cell lines revealed that F-actin assembly was abrogated through reduction of globular actin (G-actin) levels and disturbed expression of multiple actin-regulating genes. Impaired migration of these cells was associated with failure of uropod retraction likely due to altered contractility and adhesion, evidenced by reduced expression of the myosin light chain 9 (MYL9) component of myosin II complex and overexpression of CD11b integrin. Together, our results show that MKL1 is a nonredundant regulator of cytoskeleton-associated functions in immune cells and fibroblasts and that its depletion underlies a novel human primary immunodeficiency.
© 2015 by The American Society of Hematology.
Figures

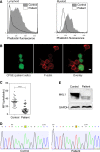


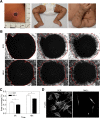


Comment in
-
Neutrophil actin regulation: MKL1 is in control.Blood. 2015 Sep 24;126(13):1519-20. doi: 10.1182/blood-2015-08-662528. Blood. 2015. PMID: 26405212 Free PMC article.
References
-
- Bouma G, Ancliff PJ, Thrasher AJ, Burns SO. Recent advances in the understanding of genetic defects of neutrophil number and function. Br J Haematol. 2010;151(4):312–326. - PubMed
-
- Kalita K, Kuzniewska B, Kaczmarek L. MKLs: co-factors of serum response factor (SRF) in neuronal responses. Int J Biochem Cell Biol. 2012;44(9):1444–1447. - PubMed
-
- Miralles F, Posern G, Zaromytidou A-I, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

