A Novel Aerosol Foam Formulation of Calcipotriol and Betamethasone Has No Impact on HPA Axis and Calcium Homeostasis in Patients With Extensive Psoriasis Vulgaris
- PMID: 26224733
- PMCID: PMC4708614
- DOI: 10.1177/1203475415597094
A Novel Aerosol Foam Formulation of Calcipotriol and Betamethasone Has No Impact on HPA Axis and Calcium Homeostasis in Patients With Extensive Psoriasis Vulgaris
Abstract
Background: Fixed combination calcipotriol 50 µg/g (Cal; as hydrate) plus betamethasone 0.5 mg/g (as dipropionate; BD) has been formulated in an innovative aerosol foam.
Objective: To assess systemic safety of Cal/BD aerosol foam.
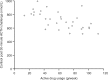
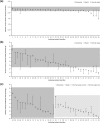
Methods: In a multicentre, single-arm, open-label, maximal-use systemic-exposure trial, adult patients with moderate to severe, extensive psoriasis (15%-30% of body surface area, including ≥30% of scalp) applied Cal/BD foam once daily. Endpoints were week 4 abnormal adrenocorticotropic hormone (ACTH) challenge test and change in albumin-corrected serum calcium, 24-hour urinary calcium excretion, and urinary calcium-creatinine ratio.
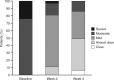
Results: 35 patients reaching week 4 exhibited normal ACTH responses. At week 4, changes in calcium homeostasis were minor and not clinically relevant; no patients experienced elevations above normal. Disease severity generally improved, and 49% of patients achieved treatment success according to the Physician's Global Assessment of Disease Severity.
Conclusion: No clinically relevant HPA axis or calcium homeostasis impact was observed with 4 weeks of once-daily Cal/BD foam in patients with extensive psoriasis vulgaris.
Keywords: dermatology; psoriasis.
© The Author(s) 2015.
Conflict of interest statement
Figures
References
-
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. - PubMed
-
- World Health Assembly. Psoriasis. Agenda item 13.5. WHA67.9. 2014. http://appswhoint/gb/ebwha/pdf_files/WHA67/A67_R9-en.pdf. Accessed February 26, 2015.
-
- Kimball AB, Gieler U, Linder D, et al. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24:989-1004. - PubMed
-
- Raho G, Koleva DM, Garattini L, et al. The burden of moderate to severe psoriasis: an overview. Pharmacoeconomics. 2012;30:1005-1013. - PubMed
-
- Hrehorow E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

