Novel Abetalipoproteinemia Missense Mutation Highlights the Importance of the N-Terminal β-Barrel in Microsomal Triglyceride Transfer Protein Function
- PMID: 26224785
- PMCID: PMC4618089
- DOI: 10.1161/CIRCGENETICS.115.001106
Novel Abetalipoproteinemia Missense Mutation Highlights the Importance of the N-Terminal β-Barrel in Microsomal Triglyceride Transfer Protein Function
Abstract
Background: The use of microsomal triglyceride transfer protein (MTP) inhibitors is limited to severe hyperlipidemias because of associated hepatosteatosis and gastrointestinal adverse effects. Comprehensive knowledge about the structure-function of MTP might help design new molecules that avoid steatosis. Characterization of mutations in MTP causing abetalipoproteinemia has revealed that the central α-helical and C-terminal β-sheet domains are important for protein disulfide isomerase binding and lipid transfer activity. Our aim was to identify and characterize mutations in the N-terminal domain to understand its function.
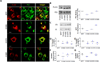
Methods and results: We identified a novel missense mutation (D169V) in a 4-month-old Turkish male child with severe signs of abetalipoproteinemia. To study the effect of this mutation on MTP function, we created mutants via site-directed mutagenesis. Although D169V was expressed in the endoplasmic reticulum and interacted with apolipoprotein B (apoB) 17, it was unable to bind protein disulfide isomerase, transfer lipids, and support apoB secretion. Computational modeling suggested that D169 could form an internal salt bridge with K187 and K189. Mutagenesis of these lysines to leucines abolished protein disulfide isomerase heterodimerization, lipid transfer, and apoB secretion, without affecting apoB17 binding. Furthermore, mutants with preserved charges (D169E, K187R, and K189R) rescued these activities.
Conclusions: D169V is detrimental because it disrupts an internal salt bridge leading to loss of protein disulfide isomerase binding and lipid transfer activities; however, it does not affect apoB binding. Thus, the N-terminal domain of MTP is also important for its lipid transfer activity.
Keywords: apolipoproteins; apolipoproteins B; cardiovascular diseases; chylomicrons; lipids; lipoproteins; microsomal triglyceride transfer protein.
© 2015 American Heart Association, Inc.
Conflict of interest statement
Figures




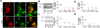


Comment in
-
Finding the Therapeutic Sweet Spot: Using Naturally Occurring Human Variants to Inform Drug Design.Circ Cardiovasc Genet. 2015 Oct;8(5):637-9. doi: 10.1161/CIRCGENETICS.115.001219. Circ Cardiovasc Genet. 2015. PMID: 26487725 No abstract available.
References
-
- Hussain MM, Bakillah A, Nayak N, Shelness GS. Amino acids 430–570 in apolipoprotein B are critical for its binding to microsomal triglyceride transfer protein. J Biol Chem. 1998;273:25612–25615. - PubMed
-
- Wu XJ, Zhou MY, Huang LS, Wetterau J, Ginsberg HN. Demonstration of a physical interaction between microsomal triglyceride transfer protein and apolipoprotein B during the assembly of ApoB-containing lipoproteins. J Biol Chem. 1996;271:10277–10281. - PubMed
-
- Patel SB, Grundy SM. Interactions between microsomal triglyceride transfer protein and apolipoprotein B within the endoplasmic reticulum in a heterologous system. J Biol Chem. 1996;271:18686–18694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

