Populus euphratica APYRASE2 Enhances Cold Tolerance by Modulating Vesicular Trafficking and Extracellular ATP in Arabidopsis Plants
- PMID: 26224801
- PMCID: PMC4577398
- DOI: 10.1104/pp.15.00581
Populus euphratica APYRASE2 Enhances Cold Tolerance by Modulating Vesicular Trafficking and Extracellular ATP in Arabidopsis Plants
Abstract
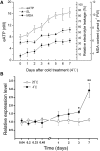
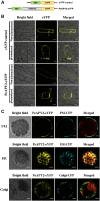
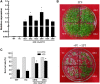
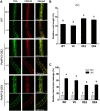
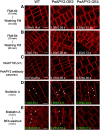
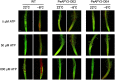
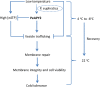
Apyrase and extracellular ATP play crucial roles in mediating plant growth and defense responses. In the cold-tolerant poplar, Populus euphratica, low temperatures up-regulate APYRASE2 (PeAPY2) expression in callus cells. We investigated the biochemical characteristics of PeAPY2 and its role in cold tolerance. We found that PeAPY2 predominantly localized to the plasma membrane, but punctate signals also appeared in the endoplasmic reticulum and Golgi apparatus. PeAPY2 exhibited broad substrate specificity, but it most efficiently hydrolyzed purine nucleotides, particularly ATP. PeAPY2 preferred Mg(2+) as a cofactor, and it was insensitive to various, specific ATPase inhibitors. When PeAPY2 was ectopically expressed in Arabidopsis (Arabidopsis thaliana), cold tolerance was enhanced, based on root growth measurements and survival rates. Moreover, under cold stress, PeAPY2-transgenic plants maintained plasma membrane integrity and showed reduced cold-elicited electrolyte leakage compared with wild-type plants. These responses probably resulted from efficient plasma membrane repair via vesicular trafficking. Indeed, transgenic plants showed accelerated endocytosis and exocytosis during cold stress and recovery. We found that low doses of extracellular ATP accelerated vesicular trafficking, but high extracellular ATP inhibited trafficking and reduced cell viability. Cold stress caused significant increases in root medium extracellular ATP. However, under these conditions, PeAPY2-transgenic lines showed greater control of extracellular ATP levels than wild-type plants. We conclude that Arabidopsis plants that overexpressed PeAPY2 could increase membrane repair by accelerating vesicular trafficking and hydrolyzing extracellular ATP to avoid excessive, cold-elicited ATP accumulation in the root medium and, thus, reduced ATP-induced inhibition of vesicular trafficking.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Bandmann V, Homann U (2012) Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J 70: 578–584 - PubMed
-
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 - PubMed
-
- Borson ND, Salo WL, Drewes LR (1992) A lock-docking oligo(dT) primer for 5′ and 3′ RACE PCR. PCR Methods Appl 2: 144–148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

