Reelin Prevents Apical Neurite Retraction during Terminal Translocation and Dendrite Initiation
- PMID: 26224852
- PMCID: PMC4518046
- DOI: 10.1523/JNEUROSCI.1629-15.2015
Reelin Prevents Apical Neurite Retraction during Terminal Translocation and Dendrite Initiation
Abstract
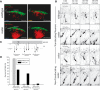
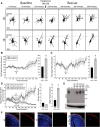
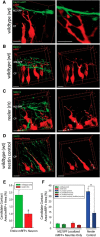
The mechanisms controlling cortical dendrite initiation and targeting are poorly understood. Multiphoton imaging of developing mouse cortex reveals that apical dendrites emerge by direct transformation of the neuron's leading process during the terminal phase of neuronal migration. During this ∼110 min period, the dendritic arbor increases ∼2.5-fold in size and migration arrest occurs below the first stable branch point in the developing arbor. This dendritic outgrowth is triggered at the time of leading process contact with the marginal zone (MZ) and occurs primarily by neurite extension into the extracellular matrix of the MZ. In reeler cortices that lack the secreted glycoprotein Reelin, a subset of neurons completed migration but then retracted and reorganized their arbor in a tangential direction away from the MZ soon after migration arrest. For these reeler neurons, the tangential oriented primary neurites were longer lived than the radially oriented primary neurites, whereas the opposite was true of wild-type (WT) neurons. Application of Reelin protein to reeler cortices destabilized tangential neurites while stabilizing radial neurites and stimulating dendritic growth in the MZ. Therefore, Reelin functions as part of a polarity signaling system that links dendritogenesis in the MZ with cellular positioning and cortical lamination.
Significance statement: Whether the apical dendrite emerges by transformation of the leading process of the migrating neuron or emerges de novo after migration is completed is unclear. Similarly, it is not clear whether the secreted glycoprotein Reelin controls migration and dendritic growth as related or separate processes. Here, multiphoton microscopy reveals the direct transformation of the leading process into the apical dendrite. This transformation is coupled to the successful completion of migration and neuronal soma arrest occurs below the first stable branch point of the nascent dendrite. Deficiency in Reelin causes the forming dendrite to avoid its normal target area and branch aberrantly, leading to improper cellular positioning. Therefore, this study links Reelin-dependent dendritogenesis with migration arrest and cortical lamination.
Keywords: dendritogenesis; lissencephaly; mental retardation; polarity.
Copyright © 2015 O'Dell et al.
Figures



References
-
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
