Function and Circuitry of VIP+ Interneurons in the Mouse Retina
- PMID: 26224854
- PMCID: PMC4518048
- DOI: 10.1523/JNEUROSCI.0222-15.2015
Function and Circuitry of VIP+ Interneurons in the Mouse Retina
Abstract
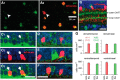
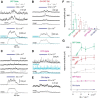
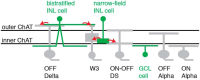
Visual processing in the retina depends on coordinated signaling by interneurons. Photoreceptor signals are relayed to ∼20 ganglion cell types through a dozen excitatory bipolar interneurons, each responsive to light increments (ON) or decrements (OFF). ON and OFF bipolar cell pathways become tuned through specific connections with inhibitory interneurons: horizontal and amacrine cells. A major obstacle for understanding retinal circuitry is the unknown function of most of the ∼30-40 amacrine cell types, each of which synapses onto a subset of bipolar cell terminals, ganglion cell dendrites, and other amacrine cells. Here, we used a transgenic mouse line in which vasoactive intestinal polypeptide-expressing (VIP+) GABAergic interneurons express Cre recombinase. Targeted whole-cell recordings of fluorescently labeled VIP+ cells revealed three predominant types: wide-field bistratified and narrow-field monostratified cells with somas in the inner nuclear layer (INL) and medium-field monostratified cells with somas in the ganglion cell layer (GCL). Bistratified INL cells integrated excitation and inhibition driven by both ON and OFF pathways with little spatial tuning. Narrow-field INL cells integrated excitation driven by the ON pathway and inhibition driven by both pathways, with pronounced hyperpolarizations at light offset. Monostratified GCL cells integrated excitation and inhibition driven by the ON pathway and showed center-surround spatial tuning. Optogenetic experiments showed that, collectively, VIP+ cells made strong connections with OFF δ, ON-OFF direction-selective, and W3 ganglion cells but weak, inconsistent connections with ON and OFF α cells. Revealing VIP+ cell morphologies, receptive fields and synaptic connections advances our understanding of their role in visual processing.
Significance statement: The retina is a model system for understanding nervous system function. At the first stage, rod and cone photoreceptors encode light and communicate with a complex network of interneurons. These interneurons drive the responses of ganglion cells, which form the optic nerve and transmit visual information to the brain. Presently, we lack information about many of the retina's inhibitory amacrine interneurons. In this study, we used genetically modified mice to study the light responses and intercellular connections of specific amacrine cell types. The results show diversity in the shape and function of the studied amacrine cells and elucidate their connections with specific types of ganglion cell. The findings advance our understanding of the cellular basis for retinal function.
Keywords: amacrine cell; optogenetics; receptive field; retinal circuitry; transgenic mice; vasoactive intenstinal polypeptide.
Copyright © 2015 the authors 0270-6474/15/3510685-16$15.00/0.
Figures







Similar articles
-
Convergence and Divergence of CRH Amacrine Cells in Mouse Retinal Circuitry.J Neurosci. 2018 Apr 11;38(15):3753-3766. doi: 10.1523/JNEUROSCI.2518-17.2018. Epub 2018 Mar 23. J Neurosci. 2018. PMID: 29572434 Free PMC article.
-
Inhibitory Interneurons in the Retina: Types, Circuitry, and Function.Annu Rev Vis Sci. 2017 Sep 15;3:1-24. doi: 10.1146/annurev-vision-102016-061345. Epub 2017 Jun 15. Annu Rev Vis Sci. 2017. PMID: 28617659 Review.
-
Morphology and function of three VIP-expressing amacrine cell types in the mouse retina.J Neurophysiol. 2015 Oct;114(4):2431-8. doi: 10.1152/jn.00526.2015. Epub 2015 Aug 26. J Neurophysiol. 2015. PMID: 26311183 Free PMC article.
-
Multiple cell types form the VIP amacrine cell population.J Comp Neurol. 2019 Jan 1;527(1):133-158. doi: 10.1002/cne.24234. Epub 2017 May 28. J Comp Neurol. 2019. PMID: 28472856
-
Bipolar Cell Pathways in the Vertebrate Retina.2007 May 24 [updated 2012 Jan 20]. In: Kolb H, Fernandez E, Jones B, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City (UT): University of Utah Health Sciences Center; 1995–. 2007 May 24 [updated 2012 Jan 20]. In: Kolb H, Fernandez E, Jones B, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City (UT): University of Utah Health Sciences Center; 1995–. PMID: 21413382 Free Books & Documents. Review.
Cited by
-
Relevance of Peptide Homeostasis in Metabolic Retinal Degenerative Disorders: Curative Potential in Genetically Modified Mice.Front Pharmacol. 2022 Jan 13;12:808315. doi: 10.3389/fphar.2021.808315. eCollection 2021. Front Pharmacol. 2022. PMID: 35095518 Free PMC article. Review.
-
Modular interneuron circuits control motion sensitivity in the mouse retina.Nat Commun. 2023 Nov 27;14(1):7746. doi: 10.1038/s41467-023-43382-0. Nat Commun. 2023. PMID: 38008788 Free PMC article.
-
Distinct inhibitory pathways control velocity and directional tuning in the mouse retina.Curr Biol. 2022 May 23;32(10):2130-2143.e3. doi: 10.1016/j.cub.2022.03.054. Epub 2022 Apr 7. Curr Biol. 2022. PMID: 35395192 Free PMC article.
-
Kir5.1 channels: potential role in epilepsy and seizure disorders.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C706-C717. doi: 10.1152/ajpcell.00235.2022. Epub 2022 Jul 18. Am J Physiol Cell Physiol. 2022. PMID: 35848616 Free PMC article. Review.
-
Nonlinear spatial integration allows the retina to detect the sign of defocus in natural scenes.Sci Adv. 2025 Aug 8;11(32):eadq6320. doi: 10.1126/sciadv.adq6320. Epub 2025 Aug 8. Sci Adv. 2025. PMID: 40779628 Free PMC article.
References
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/S0896-6273(00)00062-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
