Trib3 Is Elevated in Parkinson's Disease and Mediates Death in Parkinson's Disease Models
- PMID: 26224857
- PMCID: PMC4518050
- DOI: 10.1523/JNEUROSCI.0614-15.2015
Trib3 Is Elevated in Parkinson's Disease and Mediates Death in Parkinson's Disease Models
Abstract
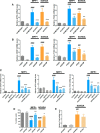
Parkinson's disease (PD) is characterized by the progressive loss of select neuronal populations, but the prodeath genes mediating the neurodegenerative processes remain to be fully elucidated. Trib3 (tribbles pseudokinase 3) is a stress-induced gene with proapoptotic activity that was previously described as highly activated at the transcriptional level in a 6-hydroxydopamine (6-OHDA) cellular model of PD. Here, we report that Trib3 immunostaining is elevated in dopaminergic neurons of the substantia nigra pars compacta (SNpc) of human PD patients. Trib3 protein is also upregulated in cellular models of PD, including neuronal PC12 cells and rat dopaminergic ventral midbrain neurons treated with 6-OHDA, 1-methyl-4-phenylpyridinium (MPP+), or α-synuclein fibrils (αSYN). In the toxin models, Trib3 induction is substantially mediated by the transcription factors CHOP and ATF4. Trib3 overexpression is sufficient to promote neuronal death; conversely, Trib3 knockdown protects neuronal PC12 cells as well as ventral midbrain dopaminergic neurons from 6-OHDA, MPP+, or αSYN. Mechanism studies revealed that Trib3 physically interacts with Parkin, a prosurvival protein whose loss of function is associated with PD. Elevated Trib3 reduces Parkin expression in cultured cells; and in the SNpc of PD patients, Parkin levels are reduced in a subset of dopaminergic neurons expressing high levels of Trib3. Loss of Parkin at least partially mediates the prodeath actions of Trib3 in that Parkin knockdown in cellular PD models abolishes the protective effect of Trib3 downregulation. Together, these findings identify Trib3 and its regulatory pathways as potential targets to suppress the progression of neuron death and degeneration in PD.
Significance statement: Parkinson's disease (PD) is the most common neurodegenerative movement disorder. Current treatments ameliorate symptoms, but not the underlying neuronal death. Understanding the core neurodegenerative processes in PD is a prerequisite for identifying new therapeutic targets and, ultimately, curing this disease. Here, we describe a novel pathway involving the proapoptotic protein Trib3 in neuronal death associated with PD. These findings are supported by data from multiple cellular models of PD and by immunostaining of postmortem PD brains. Upstream, Trib3 is induced by the transcription factors ATF4 and CHOP; and downstream, Trib3 interferes with the PD-associated prosurvival protein Parkin to mediate death. These findings establish this new pathway as a potential and promising therapeutic target for treatment of PD.
Keywords: ATF4; CHOP; Parkin; Parkinson's disease; Trib3; cell death.
Copyright © 2015 the authors 0270-6474/15/3510732-19$15.00/0.
Figures











References
-
- Bromati CR, Lellis-Santos C, Yamanaka TS, Nogueira TC, Leonelli M, Caperuto LC, Gorjão R, Leite AR, Anhê GF, Bordin S. UPR induces transient burst of apoptosis in islets of early lactating rats through reduced AKT phosphorylation via ATF4/CHOP stimulation of TRB3 expression. Am J Physiol Regul Integr Comp Physiol. 2011;300:R92-R100. doi: 10.1152/ajpregu.00169.2010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
