Differential Muscarinic Modulation in the Olfactory Bulb
- PMID: 26224860
- PMCID: PMC4518052
- DOI: 10.1523/JNEUROSCI.0099-15.2015
Differential Muscarinic Modulation in the Olfactory Bulb
Abstract
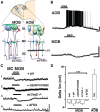
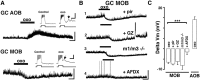
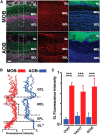
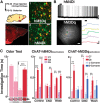
Neuromodulation of olfactory circuits by acetylcholine (ACh) plays an important role in odor discrimination and learning. Early processing of chemosensory signals occurs in two functionally and anatomically distinct regions, the main and accessory olfactory bulbs (MOB and AOB), which receive extensive cholinergic input from the basal forebrain. Here, we explore the regulation of AOB and MOB circuits by ACh, and how cholinergic modulation influences olfactory-mediated behaviors in mice. Surprisingly, despite the presence of a conserved circuit, activation of muscarinic ACh receptors revealed marked differences in cholinergic modulation of output neurons: excitation in the AOB and inhibition in the MOB. Granule cells (GCs), the most abundant intrinsic neuron in the OB, also exhibited a complex muscarinic response. While GCs in the AOB were excited, MOB GCs exhibited a dual muscarinic action in the form of a hyperpolarization and an increase in excitability uncovered by cell depolarization. Furthermore, ACh influenced the input-output relationship of mitral cells in the AOB and MOB differently showing a net effect on gain in mitral cells of the MOB, but not in the AOB. Interestingly, despite the striking differences in neuromodulatory actions on output neurons, chemogenetic inhibition of cholinergic neurons produced similar perturbations in olfactory behaviors mediated by these two regions. Decreasing ACh in the OB disrupted the natural discrimination of molecularly related odors and the natural investigation of odors associated with social behaviors. Thus, the distinct neuromodulation by ACh in these circuits could underlie different solutions to the processing of general odors and semiochemicals, and the diverse olfactory behaviors they trigger.
Significance statement: State-dependent cholinergic modulation of brain circuits is critical for several high-level cognitive functions, including attention and memory. Here, we provide new evidence that cholinergic modulation differentially regulates two parallel circuits that process chemosensory information, the accessory and main olfactory bulb (AOB and MOB, respectively). These circuits consist of remarkably similar synaptic arrangement and neuronal types, yet cholinergic regulation produced strikingly opposing effects in output and intrinsic neurons. Despite these differences, the chemogenetic reduction of cholinergic activity in freely behaving animals disrupted odor discrimination of simple odors, and the investigation of social odors associated with behaviors signaled by the Vomeronasal system.
Keywords: accessory olfactory bulb; aggression; cholinergic; muscarinic; olfactory; social behavior.
Copyright © 2015 the authors 0270-6474/15/3510773-13$15.00/0.
Figures


Similar articles
-
Cholinergic modulation of neuronal excitability in the accessory olfactory bulb.J Neurophysiol. 2010 Dec;104(6):2963-74. doi: 10.1152/jn.00446.2010. Epub 2010 Sep 22. J Neurophysiol. 2010. PMID: 20861438 Free PMC article.
-
Differential serotonergic modulation across the main and accessory olfactory bulbs.J Physiol. 2017 Jun 1;595(11):3515-3533. doi: 10.1113/JP273945. Epub 2017 Mar 31. J Physiol. 2017. PMID: 28229459 Free PMC article.
-
Distinct roles of bulbar muscarinic and nicotinic receptors in olfactory discrimination learning.J Neurosci. 2014 Aug 20;34(34):11244-60. doi: 10.1523/JNEUROSCI.1499-14.2014. J Neurosci. 2014. PMID: 25143606 Free PMC article.
-
Paying attention to smell: cholinergic signaling in the olfactory bulb.Front Synaptic Neurosci. 2014 Sep 25;6:21. doi: 10.3389/fnsyn.2014.00021. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25309421 Free PMC article. Review.
-
Vomeronasal Receptors and Signal Transduction in the Vomeronasal Organ of Mammals.In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 10. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 10. PMID: 24830038 Free Books & Documents. Review.
Cited by
-
Hyperpolarization-Activated Currents and Subthreshold Resonance in Granule Cells of the Olfactory Bulb.eNeuro. 2016 Nov 4;3(5):ENEURO.0197-16.2016. doi: 10.1523/ENEURO.0197-16.2016. eCollection 2016 Sep-Oct. eNeuro. 2016. PMID: 27844056 Free PMC article.
-
Bulbar projecting subcortical GABAergic neurons send collateral branches extensively and selectively to primary olfactory cortical regions.J Comp Neurol. 2023 Feb;531(3):451-460. doi: 10.1002/cne.25434. Epub 2022 Dec 3. J Comp Neurol. 2023. PMID: 36463397 Free PMC article.
-
Interneuron Functional Diversity in the Mouse Accessory Olfactory Bulb.eNeuro. 2019 Aug 13;6(4):ENEURO.0058-19.2019. doi: 10.1523/ENEURO.0058-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31358509 Free PMC article.
-
Lactate activates hypothalamic POMC neurons by intercellular signaling.Sci Rep. 2021 Nov 4;11(1):21644. doi: 10.1038/s41598-021-00947-7. Sci Rep. 2021. PMID: 34737351 Free PMC article.
-
Olfactory Bulb Deep Short-Axon Cells Mediate Widespread Inhibition of Tufted Cell Apical Dendrites.J Neurosci. 2017 Feb 1;37(5):1117-1138. doi: 10.1523/JNEUROSCI.2880-16.2016. Epub 2016 Dec 21. J Neurosci. 2017. PMID: 28003347 Free PMC article.
References
-
- Bloom FE, Costa E, Salmoiraghi GC. Analysis of individual rabbit olfactory bulb neuron responses to microelectrophoresis of acetylcholine norepinephrine + serotonin synergists + antagonists. J Pharmacol Exp Ther. 1964;146:16–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
