Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain
- PMID: 26224864
- PMCID: PMC6605112
- DOI: 10.1523/JNEUROSCI.0575-15.2015
Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain
Abstract
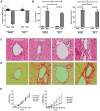
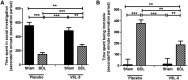
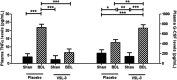
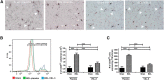
Patients with systemic inflammatory diseases (e.g., rheumatoid arthritis, inflammatory bowel disease, chronic liver disease) commonly develop debilitating symptoms (i.e., sickness behaviors) that arise from changes in brain function. The microbiota-gut-brain axis alters brain function and probiotic ingestion can influence behavior. However, how probiotics do this remains unclear. We have previously described a novel periphery-to-brain communication pathway in the setting of peripheral organ inflammation whereby monocytes are recruited to the brain in response to systemic TNF-α signaling, leading to microglial activation and subsequently driving sickness behavior development. Therefore, we investigated whether probiotic ingestion (i.e., probiotic mixture VSL#3) alters this periphery-to-brain communication pathway, thereby reducing subsequent sickness behavior development. Using a well characterized mouse model of liver inflammation, we now show that probiotic (VSL#3) treatment attenuates sickness behavior development in mice with liver inflammation without affecting disease severity, gut microbiota composition, or gut permeability. Attenuation of sickness behavior development was associated with reductions in microglial activation and cerebral monocyte infiltration. These events were paralleled by changes in markers of systemic immune activation, including decreased circulating TNF-α levels. Our observations highlight a novel pathway through which probiotics mediate cerebral changes and alter behavior. These findings allow for the potential development of novel therapeutic interventions targeted at the gut microbiome to treat inflammation-associated sickness behaviors in patients with systemic inflammatory diseases.
Significance statement: This research shows that probiotics, when eaten, can improve the abnormal behaviors (including social withdrawal and immobility) that are commonly associated with inflammation. Probiotics are able to cause this effect within the body by changing how the immune system signals the brain to alter brain function. These findings broaden our understanding of how probiotics may beneficially affect brain function in the context of inflammation occurring within the body and may open potential new therapeutic alternatives for the treatment of these alterations in behavior that can greatly affect patient quality of life.
Keywords: TNF-alpha; cerebral intravital microscopy; microglia; monocytes.
Copyright © 2015 the authors 0270-6474/15/3510822-10$15.00/0.
Figures

References
-
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
