Pravastatin Therapy and Biomarker Changes in Children and Young Adults with Autosomal Dominant Polycystic Kidney Disease
- PMID: 26224879
- PMCID: PMC4559520
- DOI: 10.2215/CJN.11331114
Pravastatin Therapy and Biomarker Changes in Children and Young Adults with Autosomal Dominant Polycystic Kidney Disease
Abstract
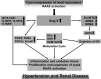
Background and objectives: Disease-specific treatment options for autosomal dominant polycystic kidney disease are limited. Clinical intervention early in life is likely to have the greatest effect. In a 3-year randomized double-blind placebo-controlled phase 3 clinical trial, the authors recently showed that pravastatin decreased height-corrected total kidney volume (HtTKV) progression of structural kidney disease over a 3-year period. However, the underlying mechanisms have not been elucidated.
Design, setting, participants, & measurements: Participants were recruited nationally from July 2007 through October 2009. Plasma and urine samples collected at baseline, 18 months, and 36 months from 91 pediatric patients enrolled in the above-mentioned clinical trial were subjected to mass spectrometry-based biomarker analysis. Changes in biomarkers over 3 years were compared between placebo and pravastatin-treated groups. Linear regression was used to evaluate the changes in biomarkers with the percent change in HtTKV over 3 years.
Results: Changes in plasma concentrations of proinflammatory and oxidative stress markers (9- hydroxyoctadecadienoic acid, 13-hydroxyoctadecadienoic acid, and 15-hydroxyeicosatetraenoic acid [HETE]) over 3 years were significantly different between the placebo and pravastatin-treated groups, with the pravastatin group showing a lower rate of biomarker increase. Urinary 8-HETE, 9-HETE, and 11-HETE were positively associated with the changes in HtTKV in the pravastatin group.
Conclusions: Pravastatin therapy diminished the increase of cyclooxygenase- and lipoxygenase-derived plasma lipid mediators. The identified biomarkers and related molecular pathways of inflammation and endothelial dysfunction may present potential targets for monitoring of disease severity and therapeutic intervention of autosomal dominant polycystic kidney disease.
Keywords: angiotensin-converting enzyme inhibitors; autosomal dominant polycystic kidney disease; oxidative stress; pediatric nephrology; statins.
Copyright © 2015 by the American Society of Nephrology.
Figures
References
-
- Parfrey PS, Bear JC, Morgan J, Cramer BC, McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S, Reeders ST: The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med 323: 1085–1090, 1990 - PubMed
-
- Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA: Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 59: 1654–1662, 2001 - PubMed
-
- Brosnahan GM: Volume progression in polycystic kidney disease. N Engl J Med 355: 733, author reply 733–734, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

