A Model-Based Illustrative Exploratory Approach to Optimize the Dosing of Peg-IFN/RBV in Cirrhotic Hepatitis C Patients Treated With Triple Therapy
- PMID: 26225222
- PMCID: PMC4369757
- DOI: 10.1002/psp4.8
A Model-Based Illustrative Exploratory Approach to Optimize the Dosing of Peg-IFN/RBV in Cirrhotic Hepatitis C Patients Treated With Triple Therapy
Abstract
Hézode et al. recently reported the frequent occurrence of anemia and thrombocytopenia in the ANRS-CO20-CUPIC cohort of hepatitis C virus (HCV) cirrhotic experienced patients treated with pegylated-interferon (Peg-IFN), ribavirin (RBV), and telaprevir or boceprevir.1,2 Using frequent measurements of serum drug concentrations, hemoglobin, and platelet concentrations obtained in 15 patients of this cohort, we show how an on-treatment model-based approach could be used to individualize dose regimen and avoid the occurrence of RBV-induced anemia and Peg-IFN-induced thrombocytopenia.
Figures
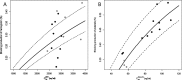
 ) and (b) Peg-IFN predicted trough concentration at steady state (Css) and predicted blocking production of platelets (
) and (b) Peg-IFN predicted trough concentration at steady state (Css) and predicted blocking production of platelets ( ). Nine patients in the telaprevir group in a (black) and six patients in the boceprevir group (gray). Eleven patients in the Peg-IFN-α2a group in b (black) and three patients in the Peg-IFN-α2b group (gray). The lines denote the predictions with the mean blocking production and the dotted lines denote 95% confidence interval computed with the standard errors predicted by the Fisher Information Matrix.
). Nine patients in the telaprevir group in a (black) and six patients in the boceprevir group (gray). Eleven patients in the Peg-IFN-α2a group in b (black) and three patients in the Peg-IFN-α2b group (gray). The lines denote the predictions with the mean blocking production and the dotted lines denote 95% confidence interval computed with the standard errors predicted by the Fisher Information Matrix.References
-
- Hézode C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J. Hepatol. 2013;59:434–441. - PubMed
-
- Hézode C, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132–142. - PubMed
-
- Laouénan C, et al. Using pharmacokinetic and viral kinetic modeling to estimate the antiviral effectiveness of telaprevir, boceprevir, and pegylated interferon during triple therapy in treatment-experienced hepatitis C virus-infected cirrhotic patients. Antimicrob. Agents Chemother. 2014;58:5332–5341. - PMC - PubMed
-
- Poordad F, et al. Effects of ribavirin dose reduction vs. erythropoietin for boceprevir-related anemia in patients with chronic hepatitis C virus genotype 1 infection––a randomized trial. Gastroenterology. 2013;145:1035–1044. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

