Physiologically Based Pharmacokinetic Modeling of Fluorescently Labeled Block Copolymer Nanoparticles for Controlled Drug Delivery in Leukemia Therapy
- PMID: 26225236
- PMCID: PMC4394613
- DOI: 10.1002/psp4.13
Physiologically Based Pharmacokinetic Modeling of Fluorescently Labeled Block Copolymer Nanoparticles for Controlled Drug Delivery in Leukemia Therapy
Abstract
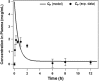
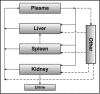
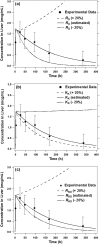
A physiologically based pharmacokinetic (PBPK) model was developed that describes the concentration and biodistribution of fluorescently labeled nanoparticles in mice used for the controlled delivery of dexamethasone in acute lymphoblastic leukemia (ALL) therapy. The simulated data showed initial spikes in nanoparticle concentration in the liver, spleen, and kidneys, whereas concentration in plasma decreased rapidly. These simulation results were consistent with previously published in vivo data. At shorter time scales, the simulated data predicted decrease of nanoparticles from plasma with concomitant increase in the liver, spleen, and kidneys before decaying at longer timepoints. Interestingly, the simulated data predicted an unaccounted accumulation of about 50% of the injected dose of nanoparticles. Incorporation of an additional compartment into the model justified the presence of unaccounted nanoparticles in this compartment. Our results suggest that the proposed PBPK model can be an excellent tool for prediction of optimal dose of nanoparticle-encapsulated drugs for cancer treatment.
Figures



References
-
- Bischoff KB, Dedrick RL, Zaharko DS. Longstreth JA. Methotrexate pharmacokinetics. J Pharm. Sci. 1971;60:1128–1133. - PubMed
-
- Bischoff KB. Physiological pharmacokinetics. Bull. Math. Biol. 1986;48:309–322. ) - PubMed
-
- Li M, Al-Jamal KT, Kostarelos K. Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano. 2010;4:6303–6317. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

