Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates
- PMID: 26225803
- PMCID: PMC4546558
- DOI: 10.1021/acs.joc.5b01343
Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates
Abstract
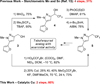
Trifluoroethylarenes are found in a variety of biologically active molecules, and strategies for accessing this substructure are important for developing therapeutic candidates and biological probes. Trifluoroethylarenes can be directly accessed via nucleophilic trifluoromethylation of benzylic electrophiles; however, current catalytic methods do not effectively transform electron-deficient substrates and heterocycles. To address this gap, we report a Cu-catalyzed decarboxylative trifluoromethylation of benzylic bromodifluoroacetates. To account for the tolerance of sensitive functional groups, we propose an inner-sphere mechanism of decarboxylation.
Figures


References
-
- Hiyama T. Organofluorine Compounds: Chemistry and Applications. New York: Springer; 2000.
- Kirsch P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications. Weinheim: Wiley-VCH; 2004.
- Bégué JP, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Weinheim: Wiley-VCH; 2008.
- Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Chichester: Wiley-Blackwell; 2009.
- Gouverneur V, Muller K. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications. London: Imperial College Press; 2012.
-
-
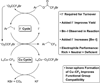
The data from SciFinder were obtained March, 2015 by conducting a substructure search for trifluoroethyl(hetero)arenes with accompanying biological studies.
-
-
-
For discussions on strategies for the preparation of trifluoroethylarenes, see: Qiao Y, Si T, Yang M-H, Altman RA. J. Org. Chem. 2014;79:7122. Kawai H, Furukawa T, Nomura Y, Tokunaga E, Shibata N. Org. Lett. 2011;13:3596.
-
-
- Kobayashi Y, Yamamoto K, Kumadaki F. Tetrahedron Lett. 1979;20:4071.
- Kondratenko NV, Vechirko EP, Yagupolskh FM. Synthesis. 1980;11:932.
- Urata H, Fuchikami T. Tetrahedron Lett. 1991;32:91.
- Duan J-X, Su D-B, Chen Q-J. J. Fluorine Chem. 1993;61:279.
- Chen Q-Y, Duan J-X. J. Chem. Soc., Chem. Commun. 1993:1389.
- Duan J-X, Chen Q-Y. J. Chem. Soc. Perkin Trans. 1. 1994:725.
- Kim J, Shreeve JM. Org. Biomol. Chem. 2004;2:2728. - PubMed
- Dubinina GG, Furutachi H, Vicic DA. J. Am. Chem. Soc. 2008;130:8600. - PubMed
- Dubinina GG, Ogikubo J, Vicic DA. Organometallics. 2008;27:6233.
- Kremlev MM, Mushta AF, Fyrra W, Yagupolskii YF, Naumann D, Möeller A. J. Fluorine Chem. 2012;133:67.
- Jiang X, Qing FF. Beilstein. J. Org. Chem. 2013;9:2862. - PMC - PubMed
- Zhu F, Fm S, Douglas JF, Altman RA. Chem. Eur. J. 2013;19:12800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

