Preparation of Inactivated Human Skin Using High Hydrostatic Pressurization for Full-Thickness Skin Reconstruction
- PMID: 26226373
- PMCID: PMC4520601
- DOI: 10.1371/journal.pone.0133979
Preparation of Inactivated Human Skin Using High Hydrostatic Pressurization for Full-Thickness Skin Reconstruction
Abstract
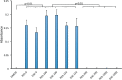
We have reported that high-hydrostatic-pressure (HHP) technology is safe and useful for producing various kinds of decellularized tissue. However, the preparation of decellularized or inactivated skin using HHP has not been reported. The objective of this study was thus to prepare inactivated skin from human skin using HHP, and to explore the appropriate conditions of pressurization to inactivate skin that can be used for skin reconstruction. Human skin samples of 8 mm in diameter were packed in bags filled with normal saline solution (NSS) or distilled water (DW), and then pressurized at 0, 100, 150, 200 and 1000 MPa for 10 minutes. The viability of skin after HHP was evaluated using WST-8 assay. Outgrowth cells from pressurized skin and the viability of pressurized skin after cultivation for 14 days were also evaluated. The pressurized skin was subjected to histological evaluation using hematoxylin and eosin staining, scanning electron microscopy (SEM), immunohistochemical staining of type IV collagen for the basement membrane of epidermis and capillaries, and immunohistochemical staining of von Willebrand factor (vWF) for capillaries. Then, human cultured epidermis (CE) was applied on the pressurized skin and implanted into the subcutis of nude mice; specimens were subsequently obtained 14 days after implantation. Skin samples pressurized at more than 200 MPa were inactivated in both NSS and DW. The basement membrane and capillaries remained intact in all groups according to histological and immunohistological evaluations, and collagen fibers showed no apparent damage by SEM. CE took on skin pressurized at 150 and 200 MPa after implantation, whereas it did not take on skin pressurized at 1000 MPa. These results indicate that human skin could be inactivated after pressurization at more than 200 MPa, but skin pressurized at 1000 MPa had some damage to the dermis that prevented the taking of CE. Therefore, pressurization at 200 MPa is optimal for preparing inactivated skin that can be used for skin reconstruction.
Conflict of interest statement
Figures








References
-
- Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

