Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer
- PMID: 26226451
- PMCID: PMC4654680
- DOI: 10.1016/j.jhep.2015.07.008
Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer
Abstract
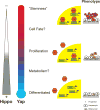
The Hippo pathway and its regulatory target, YAP, has recently emerged as an important biochemical signaling pathway that tightly governs epithelial tissue growth. Initially defined in Drosophilia, this pathway has shown remarkable conservation in vertebrate systems with many components of the Hippo/YAP pathway showing biochemical and functional conservation. The liver is particularly sensitive to changes in Hippo/YAP signaling with rapid increases in liver size becoming manifest on the order of days to weeks after perturbation. The first identified direct targets of Hippo/YAP signaling were pro-proliferative and anti-apoptotic gene programs, but recent work has now implicated this pathway in cell fate choice, stem cell maintenance/renewal, epithelial to mesenchymal transition, and oncogenesis. The mechanisms by which Hippo/YAP signaling is changed endogenously are beginning to come to light as well as how this pathway interacts with other signaling pathways, and important details for designing new therapeutic interventions. This review focuses on the known roles for Hippo/YAP signaling in the liver and promising avenues for future study.
Keywords: Hippo signaling; Liver regeneration; Stem cell biology.
Copyright © 2015 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. - PubMed
-
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. - PubMed
-
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

