Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage
- PMID: 26226598
- PMCID: PMC4635312
- DOI: 10.1016/j.jinf.2015.07.007
Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage
Abstract
Objectives: Neisseria meningitidis is a leading cause of meningitis and septicaemia. The hyperinvasive ST-11 clonal complex (cc11) caused serogroup C (MenC) outbreaks in the US military in the 1960s and UK universities in the 1990s, a global Hajj-associated serogroup W (MenW) outbreak in 2000-2001, and subsequent MenW epidemics in sub-Saharan Africa. More recently, endemic MenW disease has expanded in South Africa, South America and the UK, and MenC cases have been reported among European and North American men who have sex with men (MSM). Routine typing schemes poorly resolve cc11 so we established the population structure at genomic resolution.
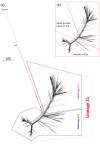
Methods: Representatives of these episodes and other geo-temporally diverse cc11 meningococci (n = 750) were compared across 1546 core genes and visualised on phylogenetic networks.
Results: MenW isolates were confined to a distal portion of one of two main lineages with MenB and MenC isolates interspersed elsewhere. An expanding South American/UK MenW strain was distinct from the 'Hajj outbreak' strain and a closely related endemic South African strain. Recent MenC isolates from MSM in France and the UK were closely related but distinct.
Conclusions: High resolution 'genomic' multilocus sequence typing is necessary to resolve and monitor the spread of diverse cc11 lineages globally.
Keywords: Genome; Meningococcal; ST-11 clonal complex; Serogroup C; Serogroup W.
Crown Copyright © 2015. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Early evidence of expanding W ST-11 CC meningococcal incidence in Spain.J Infect. 2016 Sep;73(3):296-7. doi: 10.1016/j.jinf.2016.06.010. Epub 2016 Jul 5. J Infect. 2016. PMID: 27387450 No abstract available.
-
Clinical presentation and outcome of twenty cases of Invasive Meningococcal Disease due to Serogroup C - Clonal complex 11 in the Florence province, Italy, 2015-2016.J Infect. 2017 Feb;74(2):210-213. doi: 10.1016/j.jinf.2016.12.001. Epub 2016 Dec 8. J Infect. 2017. PMID: 27940176 No abstract available.
References
-
- Halperin S.A., Bettinger J.A., Greenwood B., Harrison L.H., Jelfs J., Ladhani S.N. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl. 2):B26–B36. - PubMed
-
- Cohn A.C., Harrison L.H. Meningococcal vaccines: current issues and future strategies. Drugs. 2013;73(11):1147–1155. - PubMed
-
- Jolley K.A., Brehony C., Maiden M.C. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol Rev. 2007;31(1):89–96. - PubMed
-
- Trotter C.L., Fox A.J., Ramsay M.E., Sadler F., Gray S.J., Mallard R. Fatal outcome from meningococcal disease–an association with meningococcal phenotype but not with reduced susceptibility to benzylpenicillin. J Med Microbiol. 2002;51(10):855–860. - PubMed
-
- Brundage J.F., Zollinger W.D. Evolution of meningococcal disease epidemiology in the US army. In: Vedros N.A., editor. Evolution of meningococcal disease. CRC Press; Boca Raton: 1987.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

