KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia
- PMID: 26227020
- PMCID: PMC4521366
- DOI: 10.1186/s12990-015-0048-8
KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia
Abstract
Background: Hyperexcitability of nociceptive afferent fibers is an underlying mechanism of neuropathic pain and ion channels involved in neuronal excitability are potentially therapeutic targets. KCNQ channels, a subfamily of voltage-gated K(+) channels mediating M-currents, play a key role in neuronal excitability. It is unknown whether KCNQ channels are involved in the excitability of nociceptive cold-sensing trigeminal afferent fibers and if so, whether they are therapeutic targets for orofacial cold hyperalgesia, an intractable trigeminal neuropathic pain.
Methods: Patch-clamp recording technique was used to study M-currents and neuronal excitability of cold-sensing trigeminal ganglion neurons. Orofacial operant behavioral assessment was performed in animals with trigeminal neuropathic pain induced by oxaliplatin or by infraorbital nerve chronic constrictive injury.
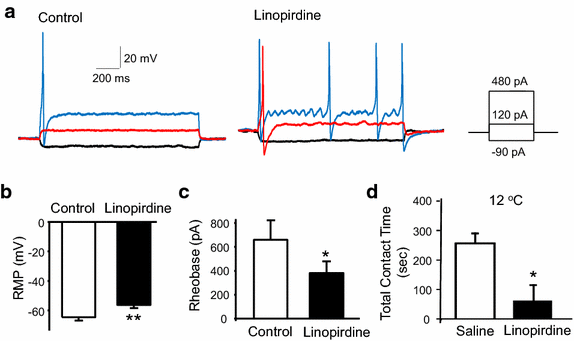
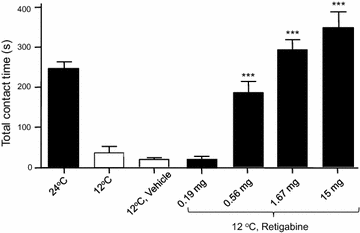
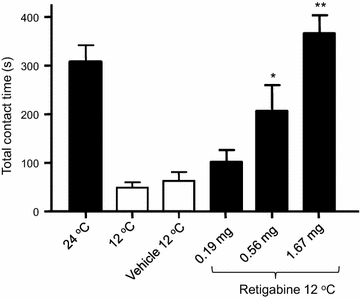
Results: We showed that KCNQ channels were expressed on and mediated M-currents in rat nociceptive cold-sensing trigeminal ganglion (TG) neurons. The channels were involved in setting both resting membrane potentials and rheobase for firing action potentials in these cold-sensing TG neurons. Inhibition of KCNQ channels by linopirdine significantly decreased resting membrane potentials and the rheobase of these TG neurons. Linopirdine directly induced orofacial cold hyperalgesia when the KCNQ inhibitor was subcutaneously injected into rat orofacial regions. On the other hand, retigabine, a KCNQ channel potentiator, suppressed the excitability of nociceptive cold-sensing TG neurons. We further determined whether KCNQ channel could be a therapeutic target for orofacial cold hyperalgesia. Orofacial cold hyperalgesia was induced in rats either by the administration of oxaliplatin or by infraorbital nerve chronic constrictive injury. Using the orofacial operant test, we showed that retigabine dose-dependently alleviated orofacial cold hyperalgesia in both animal models.
Conclusion: Taken together, these findings indicate that KCNQ channel plays a significant role in controlling cold sensitivity and is a therapeutic target for alleviating trigeminal neuropathic pain that manifests orofacial cold hyperalgesia.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

